Color in dyes is invariably explained as a consequence of the presence of a chromophore. Since, by definition, dyes are aromatic compounds, their structure includes aryl rings, which have delocalized electron systems. These are responsible for the absorption of electromagnetic radiation of varying wavelengths, depending on the energy of the electron clouds. For this reason, chromophores do not make dyes colored in the sense that they confer on them the ability to absorb radiation. Rather, chromophores function by altering the energy in the delocalized electron cloud of the dye, and this alteration results in the compound absorbing radiation from within the visible range instead of outside it. Our eyes detect that absorption, and respond to the lack of a complete range of wavelengths by seeing color.
Chromophores
Chromophores are atomic configurations which can alter the energy in delocalised systems. They are composed of atoms joined in a sequence composed of alternating single and double bonds. Double bonds in organic compounds can be of two types. If the atoms with double bonds are not adjacent, they are termed isolated double bonds, and exist independently of other double bonds in the same molecule. If adjacent atoms have double bonds, they are termed conjugated double bonds and the bonds interact with each other. Chromophore configurations often exist as multiple units, having conjugated double bonds, and are more effective when they do so. This is due to the interaction between the double bonds, which causes partial delocalization of the electrons involved in the bonds. In this case, although specific atoms are involved in the bonds, the electrons are distributed over a larger area than the specific atoms and also involve adjacent atoms that have double bonds.
The point of this is that conjugated systems have partially delocalized electrons, and the energy in these delocalized electrons can impact on the energy of the delocalized electrons of the parent aromatic compound by extending the number of electrons involved in the system and the energy needed to keep the whole system in place.
Conjugated double bonds (red)
Another common chromophore is the nitro group. This chromophore is a nitrogen with two oxygen atoms attached. One oxygen is shown attached with a single bond, the other with a double bond. In fact, like the carbon atoms in benzene, these two oxygen atoms are attached to the nitrogen with bonds of equal strength. The extra electrons are delocalized between the three atoms.
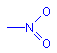
Nitro group
The quinoid ring is found in many dyes. It is a ring structure with two points at which chromophores can attach. It should be thought of as a closed system of conjugated double bonds. The attachment of configurations that add delocalized electrons to the system at one point, and the attachment of configurations that extend the atoms involved in the delocalization at the other, causes very dramatic shifts in the wavelengths that these compounds absorb. For this reason, quinoid ring configurations are considered to be extremely powerful chromophores, producing very intensely colored compounds.
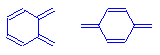
Ortho- and para- quinoid ring chromophores
To sum this up, chromophores are atomic configurations that contain delocalized electrons. Usually, they are represented as nitrogen, carbon, oxygen and sulphur that have alternate single and double bonds. By incorporating the delocalized electrons in these configurations into the delocalized electrons in the aryl rings of aromatic compounds, the energy contained in the electron cloud can be modified. If the energy incorporated into the electron cloud is changed, then the wavelength of the radiation it absorbs will also change. If this change in the wavelength to be absorbed is sufficient to cause any absorption at all within the visible range, then the compound will be colored.
Below are the usual chromophores seen in histological dyes:
–C=C–
–C=N–
–C=O–
–N=N–
–NO2
Quinoid rings
Other Effects
There is more than one effect when chemical groups are attached to aryl rings. Any delocalized electrons and their energy can simply be added to that already present, thus increasing it. Also, the delocalized electrons may be shared by more atoms than those in the original structure, by adding in to the delocalized system the atoms in the chromophore and any modifiers that may be present. Whether these effects occur jointly or separately, the final impact is an alteration in the overall energy of the electron cloud with a subsequent effect on the wavelength of radiation to which the whole molecular system reacts.
Another possibility is that electrons may be removed from the electron cloud, and this may result in loss of color. Removal of electrons may cause the remaining electrons to revert to local orbits. A good example would be Schiff’s reagent. When sulphurous acid reacts with pararosanilin, a sulphonic group attaches to the central carbon atom of the compound. This disrupts the conjugated double bond system of the quinoid ring, causing the electrons to become localized and the ring to cease being a chromophore. Consequently, the dye becomes colorless.
Auxochromes
Below are the usual auxochromes found in histological dyes:
–NH3
–COOH
–HSO3
–OH
Auxochromes are groups which attach to non ionizing compounds yet retain their ability to ionize. While this definition is largely correct, it is also inadequate. This is because it restricts the definition of the auxochrome to ionization, and does not comment on the effect of auxochromes on the absorbance of the resulting compound.
The word auxochrome is derived from two roots. The prefix auxo is from auxein, and means increased. The second part, chrome means color, so the basic meaning of the word auxochrome is color increaser. This word was coined because it was noted originally that the addition of ionizing groups resulted in a deepening and intensifying of the color of compounds.
Color Enhancing By An Auxochrome
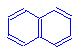
To the left is naphthalene, a colorless compound.
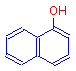
The addition of a single hydroxyl group to naphthalene produces 1-naphthol, which is also a colorless compound, but one that can ionize.
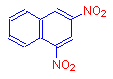
If instead of a hydroxyl group, we add the nitro group, which is a chromophore, we get the compound 2,4-dinitronaphthalene. The addition of this chromophore has caused it to become pale yellow.
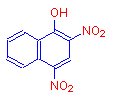
If instead of a hydroxyl or nitro groups, both a hydroxyl and nitro groups are added, we get the deep yellow dye, martius yellow.
The addition of both an auxochrome and a chromophore results in a much stronger alteration of the absorption maximum of the compound. The hydroxyl group must have deepened the color, showing that auxochromes are also chromophores.
Sometimes the term auxochromophoric is used to denote the action of an auxochrome that modifies the color as well as permitting ionization. This term infers that the color modifying effects of auxochromes are rare, but this is not the case. The effect on absorption should not be considered an incidental aspect of the auxochrome’s action, but an integral and fundamental part of it. The word auxochromophoric is redundant.
Edward Gurr proposed the terms colligator and non-colligator to distinguish between ionizing auxochromes and color modifying effects.
Auxochromes are of two types. They may have a positive charge as the amino group and its substituted variants. Or they may be negatively charged as the carboxyl and hydroxyl groups, and the sulphonic group. This last is commonly used to convert basic dyes to acid dyes. Both negatively charged and positively charged auxochromes may be present on a single molecule.
Resonance
The process by which electrons are stimulated by radiation is resonance. It should be made clear at the outset that resonance is not the same as vibration.
Resonance is the induction of a response in one energy system from another energy system in close proximity, which is operating at the same energy level (frequency). In aromatic organic compounds, including dyes, the two energy systems are electromagnetic radiation, and the delocalized electron cloud. Do not confuse resonance with the imaginary resonance hybrids used in an older explanation for the structure of benzene.
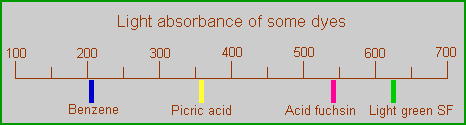
Absorption of benzene compared to some dyes
Modifiers
Color modifiers such as methyl or ethyl groups alter the color of dyes by altering the energy in the delocalized electrons. By themselves, they cannot do this enough to cause absorption in the visible range, but they can affect the shade significantly when absorption is already in that range. Adding more of a particular modifier results in a progressive alteration of color. Compounds that differ from each other in this kind of regular fashion are called homologues. A very good example is seen with the Methyl violet series.
Alteration of Color by Modifiers
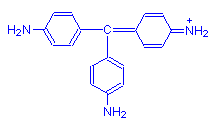
Without any methyl groups, the parent dye is called pararosanilin and is red.
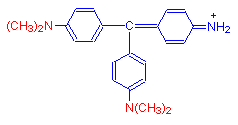
When four methyl groups are added, we get the reddish purple dye methyl violet.
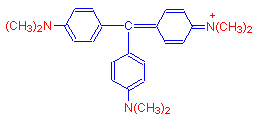
As more methyl groups are added, we get the purple blue dye crystal violet, which has six such groups.
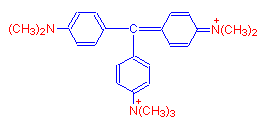
If a seventh methyl group is added, the resulting dye is methyl green.






