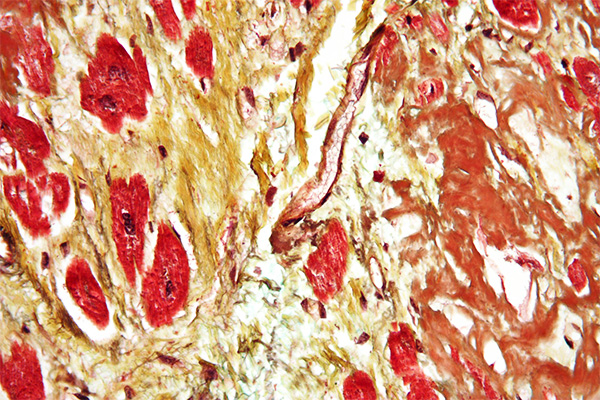
Silverman-Movat Pentachrome
for Elastic, Mucin & Collagen
Expected Results
- Nuclei – dark purple to black
- Elastic fibres – purple to black
- Muscle – Red, with longitudinal myofibrils, cross striations and intercalated discs delineated
- Collagen and bone – yellow to yellow green
- Mucin – blue
- Ground substance – blue green
- Cytoplasm – pink to brownish to red
Materials
Stock solution A
| Material | Amount | |
|---|---|---|
| Orcein | 1 | g |
| Hydrochloric acid, conc. | 1 | mL |
| Ethanol, 70% | 500 | mL |
Stock solution B
| Material | Amount | |
|---|---|---|
| Hematoxylin | 8 | g |
| Ethanol, absolute | 160 | mL |
Stock solution C
| Material | Amount | |
|---|---|---|
| Ferric chloride | 9.6 | g |
| Distilled water | 90 | mL |
Stock solution D
| Material | Amount | |
|---|---|---|
| Iodine | 1 | g |
| Potassium iodide | 2 | g |
| Distilled water | 97 | mL |
Working elastic solution
| Material | Amount | |
|---|---|---|
| Stock solution A | 25 | mL |
| Stock solution B | 8 | mL |
| Stock solution C | 5 | mL |
| Stock solution D | 5 | mL |
Differentiator
| Material | Amount | |
|---|---|---|
| Stock C | 10 | mL |
| Distilled water | 40 | mL |
Alcian blue
| Material | Amount | |
|---|---|---|
| Alcian blue | 1 | g |
| Acetic acid, glacial | 1 | mL |
| Distilled water | 99 | mL |
Ammoniated ethanol
| Material | Amount | |
|---|---|---|
| Strong ammonia | 5 | mL |
| Ethanol, 95% | 95 | mL |
Plasma stain
| Material | Amount | |
|---|---|---|
| Woodstain scarlet 1% aqu. | 4 | mL |
| Acid fuchsin 1% aqu. | 1 | mL |
| Acetic acid, 0.5% | 95 | mL |
Polyacid
| Material | Amount | |
|---|---|---|
| Phosphotungstic acid | 5 | g |
| Distilled water | 100 | mL |
Fiber stain
| Material | Amount | |
|---|---|---|
| Spanish saffron | 6 | g |
| Ethanol, absolute | 96 | mL |
Preparation
- Incubate together in a sealed container at 56°C for 48 hours. Cool.
Tissue Sample
Paraffin sections at 4-6µ are suitable. Bouin’s fixation is preferred. 10% neutral buffered formalin is satisfactory. If formalin is used, refix sections with Bouin’s fluid for one hour at 56°C, then wash in running tap water to remove the yellow colour.
Protocol
- Bring sections to water via xylene and ethanol.
- Stain in alcian blue for 20 min.
- Rinse in distilled water.
- Place in ammoniated ethanol for 10 min at 56°C.
- Wash in running tap water for 2 min.
- Rinse in distilled water.
- Stain in working elastic solution for 2 hours.
- Wash in running water until collagen is clear and elastic prominent.
- Rinse in distilled water.
- Place in the plasma stain for 3 minutes.
- Place in 0.5% aqueous acetic acid for 30 seconds.
- Differentiate in the polyacid until collagen is clear and ground substance is blue.
- Rinse in 0.5% aqueous acetic acid for 30 seconds.
- Place in three changes of absolute ethanol for 1 minute each.
- Place in the fiber stain for 8 minutes.
- Dehydrate quickly in absolute ethanol, 2 changes.
- Clear in xylene, three changes.
- Coverslip with a resinous mounting medium.
Notes
- The working elastic solution should be used only once, then discarded.
- If the elastic fibers are not clearly delineated at step 8 and the background is not clean, place in the differentiator for a few minutes, then wash well in tap water until they are sharp.
- Differentiation with the polyacid at step 12 takes from 3-10 minutes.
- The fiber stain (alcoholic saffron) may be used repeatedly until staining intensity decreases. It is important that this solution not be contaminated with water. Place some Drierite into it to keep it anhydrous.
Safety Note
Prior to handling any chemical, consult the Safety Data Sheet (SDS) for proper handling and safety precautions.
References
- Silverman, J., (1972)
Histologic v 2, N° 2.