Fibrin is a protein material derived from the fibrinogen in blood plasma. Its primary function is to stop bleeding from cuts and abrasions both internally and externally. The process is well documented, having been extensively studied for bleeding disorders. For those interested in the clotting process, during which fibrin is formed, a good hematology reference text would be the best place to start. Very briefly, the process is that a cut or an abrasion to a blood vessel occurs which cause blood platelets to react and initiate a process whose function is to convert fibrinogen to fibrin. The fibrin forms a meshwork which traps materials and forms a blood clot which plugs the cut and stops the bleeding.
Introduction
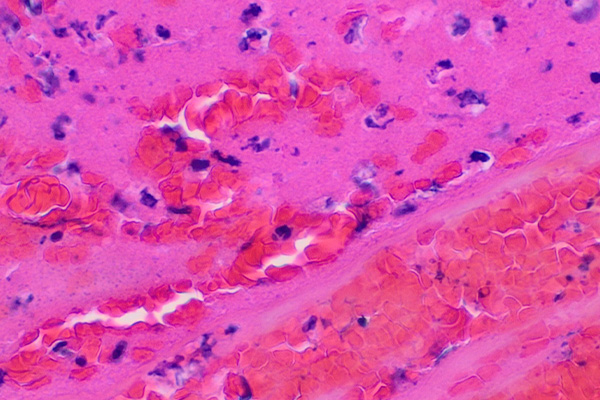
Fibrin is an acidophil material, that is, it is stained by acid dyes and is pink in an H & E. From the histological perspective, blood clots are pretty obvious in most cases, and the fibrin can often be seen in an H & E as distinctly pink but less brilliant than the erythrocytes. There are some diseases, though, which produce small amounts of fibrin within the tissues, perhaps in conjunction with a few erythrocytes, perhaps alone. These fine fibrin deposits can be in the form of fibers, small plates or granules, and it is often important for a pathologist to identify them as an aid to diagnosis. Renal biopsies, as an example, will often be stained to demonstrate fibrin deposits within the glomeruli. These may be about the size and shape of erythrocytes which may also be present, so it becomes important to be able to identify which material is an erythrocyte and which is a fibrin deposit. There is a similar difficulty if the fibrin deposit is a fine fiber. Collagen may also be present as fine fibers, and in an H & E it may be difficult to reliably differentiate between the two. Fortunately, staining methods are available to color these three materials in different colors and clearly identify them.
Staining Methods for Fibrin
The older methods for fibrin do not differentiate between fibrin and erythrocytes particularly well. Both fibrin and erythrocytes are likely to be blue stained with phosphotungstic acid hematoxylin (PTAH). If the PTAH is applied for a short period, perhaps 15 minutes or so, the fibrin may be blue and the erythrocytes unstained. This is not reliably so, however. The method is more successful with larger deposits of fairly fresh fibrin than fibrin the size of an erythrocyte that may be encountered in renal biopsies. The other traditional technique is the Weigert Gram stain variant. This is a Gram stain which is differentiated with aniline-xylene and is often done after prestaining with eosin or some other red counterstain. Fibrin retains the crystal violet, appearing deep blue on a pink background. Again, this technique is more successful with larger deposits than the erythrocyte sized ones sometimes encountered. In practice, these older methods are not often used any more and have been superseded by the trichrome staining methods introduced by Lendrum and his coworkers.
Lendrum proposed that fibrin deposits stained differently depending on their age since being formed. The freshest fibrin stains with the smallest molecular weight dyes, much like erythrocytes, then progressively with larger molecular weight dyes until it is indistinguishable from collagen. He proposed several staining techniques to identify fibrin at different stages in this aging process. Still, many of the methods demonstrate fibrin at ages between extremely fresh and very old, so for practical purposes most of the fibrin will be seen with these methods, and only rarely are the other methods required.
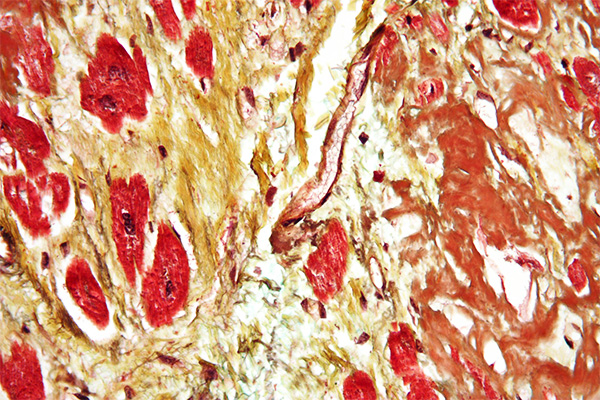
Perhaps the “type” trichrome method, as far as fibrin is concerned, is the Picro-Mallory. This technique uses three staining solutions and a polyacid to produce yellow erythrocytes, red fibrin and blue collagen. Unfortunately, the full method is time consuming and requires some experience to produce optimal results. It is unsurpassed when it does so. There is a simpler variant of the method which can give very acceptable results for most practical purposes but which still requires some experience.
Perhaps the most popular trichrome is the Martius, Scarlet and Blue (MSB). This method has become popular as it is less dependent on the skill of the technologist and the extended fixation that is desirable with the Picro-Mallory, and may be done quite adequately on formalin fixed tissues with secondary fixation of sections for an hour at 60°C in either Bouin’s fluid or saturated aqueous picric acid. It demonstrates most fibrin red, although it should be noted that the freshest fibrin is yellow and very old fibrin is blue. This is likely the most appropriate technique for routine use in diagnostic settings.
For special cases the Masson 44/41 (so called because it uses acid red 44 and acid blue 41) demonstrates older fibrin than the Picro-Mallory and MSB, and the Obadiah technique (so called from an acronym of Orange, Blue, Direct Red – OBDR) demonstrates the oldest fibrin of all, when it otherwise is indistinguishable from collagen. These two methods would not usually be used routinely.
Other techniques have been recommended, but they tend to be fairly complex and require considerable experience. Slidders’ Fuchsin-Miller is a yellowsolve method that demonstrates most fibrin bright red against a yellow background and can be quite striking. It is very effective for colour photography. The yellowsolve I technique may also be used, but is not popular.
Fibrinoid is a material resembling fibrin. The ending -oid means “like”, as in “similar to”, so fibrinoid means like fibrin. Fibrinoid is a hyaline material seen in some diseases, often autoimmune, which stains like fibrin but has other staining reactions different from fibrin. It is now generally accepted that fibrinoid is fibrin that is mixed with or which has trapped other materials which alter its staining reactions.
References
- Lendrum A C, Fraser D S, Slidders W and Henderson R. (1962)
Studies on the character and staining of fibrin.
Journal of clinical pathology, v. 15, p. 401. - McManus, J.F.A. and Mowry, R.W., (1960),
Staining methods, histologic and histochemical,
Harper & Row, New York, NY, USA. - Histological demonstration techniques, (1974)
Cook, H C.
Butterworths, London, England