Although the word trichrome actually means three color, it no longer has that specific significance. The term is used now to describe those staining methods which use two or more acid dyes of contrasting colors to selectively color different basic tissue components. Most commonly they are used to demonstrate collagen, often in contrast to smooth muscle, but may also be used to emphasize fibrin in contrast to erythrocytes. Other components can also be selectively demonstrated.
What is a Trichrome?
The pedantic meaning of trichrome is three colored. Apart from that there is no clear definition of what constitutes this kind of staining technique. A good basis for comparison is Masson’s methods, but even this does not clarify the term since in some variants he used three dyes, and in others he used only two. Some people consider the nuclear stain as one of the colors, others do not.
Since counting colors is rather pointless, the term trichrome is used in this section of StainsFile to include any staining technique meeting the following criteria:
- At least one acid dye is used.
- A polyacid is an integral part of the method:
- Phosphotungstic acid
- Phosphomolybdic acid
- Some other acid acting similarly
- The acid functions as a differentiator or inhibitor (resist) of an acid dye.
- The acid’s function can be explained by dye competition (displacement).
These criteria include methods which demonstrate tissue components such as collagen and muscle, the most common purpose, but also includes methods for fibrin, pituitary cells, and those methods which include the staining of elastic with dyes such as orcein in a solution which also stains collagen and muscle. These latter methods are often called tetrachrome or polychrome methods, but for convenience are included with the trichrome methods.
Strictly speaking, the picro-fuchsin variants, such as the van Gieson and related methods, would be excluded by these criteria. It would also exclude methods which stain with a red acid dye, then apply a large molecular weight yellow dye in an anhydrous solvent. These are the Yellowsolve Methods. However, both of these groups are included due to their being dependent on the same fundamental processes as the true trichrome methods.
How Trichrome Staining Works
The most widely accepted basis for these methods is an application of mass action, coupled with observations on the physical properties of various tissue components and their impact on staining. At the outset it must be made clear that the methods control how ionized acid dyes react with ionized basic tissues. That is the fundamental principle on which they depend, and the explanation is only about how that fundamental reaction can be manipulated.
Important Components
Following appropriate fixation and processing, sections within the range of about 3 – 8 microns are cut. The section thickness can have an effect on the results, but within that range is usually satisfactory. They are baked onto the slides, and when ready to stain they are brought to water in the usual manner.
Trichrome methods invariably use dyes in acid pH solvents, usually dilute aqueous acetic acid. Usually the concentration of acetic acid matches the concentration of dye (1% dye in 1% acid, 2% dye in 2% acid etc.). This is not necessary, but is common practice. The acid pH itself is necessary to maximize the amount of dye that will attach to tissue amino groups, i.e. it is an accentuator.
This low pH has an effect on nuclear staining with alum hematoxylin, which is either removed or appears red. This is overcome by using ferric mordanted hematoxylin, which resists removal by acids better than aluminum mordanted solutions. Probably the two commonest techniques are Weigert’s hematoxylin and the celestine blue hemalum sequence.
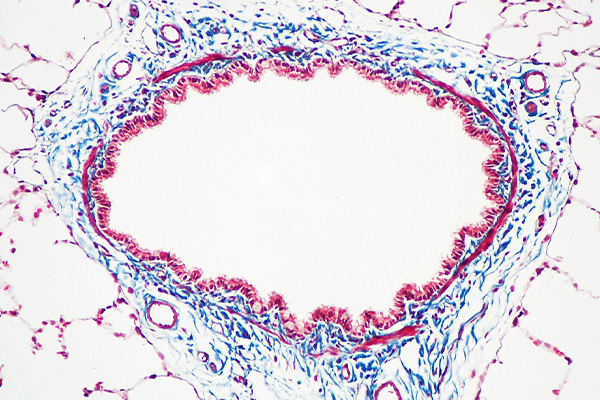
These techniques usually stain cytoplasm with a red dye, which is often called the plasma stain. Collagen is usually stained with a green or blue dye which may be called the fiber stain. Small molecular weight yellow dyes are sometimes included to stain erythrocytes so they contrast well with the red staining components, which can include fibrin. For consistency, these will be referred to as the erythrocyte stain.
See also an explanation of yellowsolve methods.
Multi-Step Methods
Multi-step techniques include methods such as the Masson’s trichrome, which is used to differentiate between smooth muscle fibers and collagen, or to demonstrate a change in the amount of collagen present. Other methods, such as Lendrum’s Picro-Mallory may be used to demonstrate fibrin in sharp contrast to erythrocytes and other tissues. In these methods the dyes are applied sequentially, and staining is optimized at each step.
To selectively stain erythrocytes, small molecular weight yellow acid dyes such as picric acid and martius yellow are dissolved in ethanol. A polyacid may also be included. The solution is applied for a period then the sections are washed in water, usually tap water, to remove excess yellow staining from tissues. The erythrocyte limiting membrane permits the entrance of water. This dilutes the ethanol increasing polarity, and results in the dye aggregate size increasing, thus trapping the dye within the erythrocytes. This step is optional, and many techniques do not incorporate it. In those cases erythrocytes stain the same color as cytoplasm.
The second dye (the plasma stain) is then applied. This is usually red, and of intermediate molecular weight. It is applied for long enough to deeply stain all of the tissue, including cytoplasm, muscle and collagen. If erythrocytes have been pre-stained with yellow dyes, they resist staining with this dye for some time. Otherwise they are also stained with it.
The polyacid is then applied. This has a large molecule, and removes (differentiates) the plasma stain from tissues. The first structures affected are collagen and bone. When it has been applied for long enough, these tissue components macroscopically appear much paler than muscle fibers or cytoplasm.
Following the polyacid, the contrast dye (the fiber stain) is applied. This has a molecular weight larger than the plasma stain, but considerably less than the polyacid. The dye is applied for long enough to strongly stain collagen fibers without beginning to replace the red staining of other components.
The most common staining pattern is:
- Erythrocytes – yellow (or red)
- Cytoplasm, fibrin, muscle – red
- Collagen, bone – green or blue
In some methods staining with the fiber stain is accentuated to increase the contrast of the target element, usually fibrin, causing muscle and cytoplasm to appear blue tinged. There are also methods with a color pattern different than above, yellow collagen for instance, or dark blue fibrin.
One-Step Methods
One-step trichrome staining methods are those that combine all of the dyes and other reagents into a single solution, which is applied for a specified time. The various tissue components are thereby coloured differentially. These methods include van Gieson’s and Gomori’s methods.
One step methods are technique dependent. They work satisfactorily provided that everything is standardized. This includes fixation, processing and section thickness as well as the formulation of the staining solution and the length of time for which it is applied. While individual techniques may be more or less tolerant of minor changes, in general, changing any of the parameters will require that the staining procedure be restandardized for consistency of results. In other words, one step methods work fine provided you don’t change the procedure at all.
By incorporating all of the reactants into a single solution the various factors interact simultaneously resulting in various tissue components staining with different dyes. The basis is the trend towards reaching equilibrium caused by the reaction products participating in a mass action type reaction. It is for this reason that one-step methods must be so standardized, as changing any one of the parameters may result in a different equilibrium.
In practice, we do not let equilibrium be reached. This nearly always favors the fiber stain and would generally be undesirable. Instead, the process is interrupted by removing the solution when the desired results are obtained. This time period is one of the standardized factors. Proper staining is obtained by interrupting the progress towards equilibrium at a specified, repeatable point.
One-step methods can be adjusted by altering any of several factors. However, this is usually a matter of trial and error. Change only one factor at a time and keep careful records.
In general, it will be found that increasing the time applied will bias the staining in favor of the fiber stain. It may also increase stain intensity. Decreasing the time, however, will bias staining in favor of the plasma stain. Erythrocyte staining is usually less affected. It may also reduce stain intensity.
Increasing the concentration of a dye and keeping the time constant will bias the staining in favor of that dye, while diminishing staining from the other dyes. In the case of the erythrocyte dye, this will often result in yellow tinged or orange cytoplasm. If it is the plasma stain, staining will be more intense in the cytoplasm and may result in some red collagen. It may also intrude into the erythrocytes causing them to stain orange, or be inconsistently colored. If the fiber stain is increased, it will intrude into cytoplasmic structures, but will not generally affect erythrocyte staining.
Decreasing the concentration of a dye while keeping the time constant will reduce staining by that dye and increase the effect from the other dyes. If the erythrocyte dye is reduced, the plasma stain will invariably stain the erythrocytes to some degree, even obscuring staining by the erythrocyte dye completely. If the plasma stain is reduced there may be some yellow coloring in some structures such as fibrin or muscle, and there may be some infiltration of the fiber stain into other structures such as cytoplasm and muscle. If reduced too much, a stain of yellow and blue with an occasional red blob results, which is particularly unattractive. Reducing the fiber stain diminishes the staining of collagen, often leaving it with a red tinge.
Altering the amount of other ingredients (type and amount of polyacid, type and concentration of solvent) will also have an impact. These should be carefully evaluated as they can be inconsistent.