Nitrocellulose, otherwise known as cellulose nitrate, goes by several names. The commonest, in the histological world, are celloidin, collodion and parloidin, but others are not infrequently encountered, many of them ending in “-oidin”. Many of these names are well established trade names for different companies’ products.
Introduction
Nitrocellulose is also known as gun cotton from its use as an explosive and its appearance, which can resemble cotton wool in the dry state and can be produced from cotton, which is made of cellulose. As would be expected of an explosive, it is extremely inflammable, a characteristic which is encreased when dissolved in a mixture of absolute ethanol and ether, both either inflammable or explosive fluids in their own right. Open flames, sparks from equipment, smoking tobacco in any form and any other activity which can cause ignition of the material or its solvent must be absolutely forbidden. Explosion safe processing and storage containers are essential, and common sense must be used at all times. Fire extinguishers, fire blankets and other fire safety equipment must be easily and conveniently available at all times. Processing with nitrocellulose is safe if these precautions are taken.
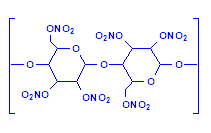
The celloidin which was originally used for histological processing produced a thick, syrupy solution when dissolved in ethanol-ether (alcohol-ether). Later a material which produced a less viscous solution for the same weight of nitrocellulose was introduced, called low viscosity nitrocellulose (LVN). This made it possible to give greater support to the tissues by using higher concentrations of nitrocellulose for the same viscosity. Both products are still in use. As you can see from the formula, celloidin is a polymer of nitrated cellulose units.
Celloidin embedding is a slow process, taking some weeks usually, and does not produce sections as thin as those produced by paraffin embedding. The minimum thickness is about 12 µ but 15 µ, or thicker, sections are often cut. Section cutting is usually done wet, which means that the block is lubricated with a fluid, usually 60-70% ethanol, and is not allowed to dry out. This makes section cutting somewhat messy and quite a bit slower than the dry sectioning used with paraffin. When sections have been cut, they are then stored in 60-70% ethanol instead of being adhered to glass slides. They are usually stained free floating and put on slides at the same time as the coverslip is applied. This does make it difficult to prepare serial sections as each section must be stored in an individual, numbered container or small batches of 5 or so sections prepared. These are not really “serial” sections but they are close. Blocks are stored in the same alcohol as used for lubrication, and they must never be allowed to become dry.
Tissues embedded in celloidin are usually sectioned with a sliding microtome. In this instrument the block is mounted on a platform facing upwards and is fixed. The knife is held at a significant slant so that most of the blade edge is used during the cutting stroke, and is quite long, often in excess of 25 cm. It is customary to use a deeply plano-concave knife, the cutting stroke being to slice off a section rather than to peel it off, so that the strength behind the knife edge is not needed but a very keen edge is necessary. The face of the block is lubricated with 70% ethanol and the knife drawn across the top of the block at a strong slant, shaving off a section, which is immediately removed and placed in 70% ethanol. The surface of the block is then relubricated for the next cutting stroke.
The desirable aspects of celloidin processing are to do with it being a process that completely avoids the use of heat at any stage. As a consequence, heat produced artefacts are avoided. In particular, shrinkage is absolutely minimal, if there is any, and organs composed of layers of materials of different characteristics do not have the layers separate or shrink away from each other so the structural relationships of the various types of tissue components can be seen clearly.
The undesirable aspects are the time it takes, the thickness of the sections, the necessity for staining to be done on free floating sections with the celloidin still present, the resulting restrictions on the variety of staining methods that may be used, the greater difficulty in final dehydration and clearing which is caused by the absolute ethanol softening the celloidin excessively, and the inconvenience of storing the blocks in sealed jars with lids tight enough to stop evaporation of 70% ethanol completely.
The thickness of the sections is considered an advantage by some and a disadvantage by others, depending on whether the microscopist wants to see multiple layers of cells and the relationships between them as the microscope is focused up and down, or a single layer without interference from surrounding material.
Making Celloidin Solutions
Celloidin is used in solution, usually in a 1:1 mixture of ethanol-ether at concentrations of 2%, 4% and 8%. The equivalent concentrations for LVN are 5%, 10% and 20%. Celloidin may be purchased either as a solution or as a solid, damped with a liquid to reduce flammability. This is usually an alcohol, so its weight may be ignored when dissolving. The stock purchased as a solution may be in an undesirable solvent and it is often the practice to evaporate the solvent to obtain dry celloidin, which is then weighed and redissolved in the appropriate solvent. The evaporation to dryness is done slowly at room temperature, without additional heat and in an explosion safe environment as a fire safety precaution.
The fastest way to dissolve celloidin is to soak it first in half the final volume of anhydrous ethanol to soften it (50 mL for each 8 grams celloidin), with intermittent mixing in a tightly stoppered container. The next day, an equal volume of diethyl ether is added and intermittently mixed until an evenly consistent solution is obtained. The 2% and 4% solutions may then be made by simple dilution of the 8% solution with an equal parts mixture of ethanol and diethyl ether. The solution should be transparent, without undissolved material, and should be stored in a completely closed container which is ether resistant. Glass is suitable.
Fixation
Celloidin is used in solution, usually in a 1:1 mixture of ethanol-ether at concentrations of 2%, 4% and 8%. The equivalent concentrations for LVN are 5%, 10% and 20%. Celloidin may be purchased either as a solution or as a solid, damped with a liquid to reduce flammability. This is usually an alcohol, so its weight may be ignored when dissolving. The stock purchased as a solution may be in an undesirable solvent and it is often the practice to evaporate the solvent to obtain dry celloidin, which is then weighed and redissolved in the appropriate solvent. The evaporation to dryness is done slowly at room temperature, without additional heat and in an explosion safe environment as a fire safety precaution.
The fastest way to dissolve celloidin is to soak it first in half the final volume of anhydrous ethanol to soften it (50 mL for each 8 grams celloidin), with intermittent mixing in a tightly stoppered container. The next day, an equal volume of diethyl ether is added and intermittently mixed until an evenly consistent solution is obtained. The 2% and 4% solutions may then be made by simple dilution of the 8% solution with an equal parts mixture of ethanol and diethyl ether. The solution should be transparent, without undissolved material, and should be stored in a completely closed container which is ether resistant. Glass is suitable.
Dehydration
Dehydration should also be thorough and complete, taking a few days with several changes of ethanol. For delicate tissues, gradual dehydration is strongly recommended to avoid distortion from removing the water too fast. Keep in mind that celloidin processing causes almost no distortion of tissue layers and the gradual increasing of the ethanol concentration is important in producing this distortion free appearance. It does mean that poorly fixed material has a longer time to deteriorate from residual moisture before the ethanol content increases sufficiently to stop it, and this makes thorough fixation important.
Infiltration
No clearing agent is used with celloidin and following dehydration with absolute ethanol, the tissue may be placed in ethanol-ether. Ether is a lipid solvent and will remove much of it from the tissue. The tissues should then be placed into the thinnest of the celloidin solutions (2%) and left in a tightly capped container for an appropriate length of time. How long is appropriate? That depends on the tissue and could be a week or months. The denser it is, the longer it must be left to infiltrate. Experience is the best judge, but care should be taken not to be impatient because, if the infiltration time is too short and the center of the block is not properly infiltrated, short of removing all of the celloidin and repeating the process from the beginning, a procedure that would take longer than the original processing, there is nothing that can be done. It is far better to allow an adequate period of time for infiltration.
This process is then repeated with the medium and thickest solutions of celloidin. Infiltration time is as long or longer than for the thinnest solution and, for the same reasons, should not be rushed.
Casting and Hardening
When infiltration is complete the block has to be cast and hardened. Paper boats do very well for this, and have the advantage that they may be cut off if the paper does not peel away easily. The instructions below for making paper boats are from The Microtomist’s Vade-Mecum.
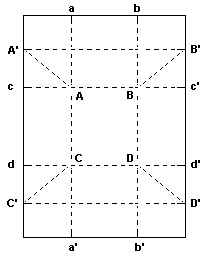
“To make paper trays proceed as follows. Take a piece of stout paper or thin cardboard, of the shape of the annexed figure; thin post-cards do very well indeed. Fold it along the lines a a’ and b b’, then along c c’ and d d’, taking care to fold always the same way.
Then make the folds A A’, B B’ , C C’, D D’, still folding the same way. To do this you apply A c against A a, and pinch out the line A A’, and so on for the remaining angles. This done, you have an imperfect tray with dogs’ ears at the angles. To finish it, turn the dogs’ ears round against the ends of the box, turn down outside the projecting flaps that remain, and pinch them down.”
Some thick celloidin is poured into the bottom of a boat, then the tissue arranged in it and more celloidin poured in to well cover the tissue, keeping in mind that as the block hardens the celloidin will shrink. If at any time the celloidin shrinks enough to expose the tissue, more celloidin should be poured in to cover it. At the end of the hardening process there should be sufficient celloidin to allow for trimming the back of the block flat so that it may act as a base for glueing the block to a wooden holder for sectioning. Usually 5 mm or more is needed.
After filling the boat with thick celloidin it is placed under a bell jar, or something similar, with a base that ensures air is excluded. A plate of glass is satisfactory. Each day the top of the jar is lifted a little for a few minutes so that evaporated ethanol-ether can escape. During the following day more solvent will evaporate from the block and the atmosphere inside the bell jar will become saturated again. Evaporating the solvent off slowly in this manner ensures that the celloidin thickens and hardens evenly throughout the tissue. Evaporating it too fast will result in the outside of the block becoming hard while the inside is still soft. Using this method of hardening the block also allows the concentration of celloidin to increase and give additional support to the tissue. The hardening process takes some time and is complete when the block is sufficiently hard, often judged by pressing it with a finger nail and having some difficulty leaving an impression.
At any time during the hardening procedure, it is possible to bring it to completion within about 24 hours or so, but with somewhat less support to the tissue since the celloidin will not have had an opportunity to properly concentrate. Simply place a small open container of chloroform under the bell jar. The chloroform will saturate the atmosphere and harden the celloidin without further evaporation.
Sectioning
Once hardened, the block is removed from the paper boat, preferably by peeling, but it can be cut away with a sharp blade if necessary. The block is then trimmed, allowing about 3 mm or so celloidin all around the tissue, then a distinctive cut is made on one corner for orientation. The back is trimmed flat. The block is then placed into 70% ethanol until ready to section.
Identifying the block with an accession number can be done by loading a sharp point with India ink, then pricking a series of holes in the side of the block, leaving some India ink in the hole. Extra ink may be rubbed over the holes to force some ink into them. After the block has been sectioned a paper label may be attached to the side with celloidin and covered.
To section, the block is first attached to a, usually wooden, holder. Place some thick celloidin onto the holder and press the block into it, arranging it so that it will meet the knife as wanted. A weight is then placed onto the block and the assembly is left until the attachment is firm. The whole thing is then placed into 70% ethanol until ready for sectioning.
Sectioning is best demonstrated and taught by an experienced technologist. It is done slowly, the sections being sliced off the block individually and immediately placed into 70% ethanol. The block surface is wetted with 70% ethanol after each section is removed, and it is never allowed to dry out. Roughing down is done in the same fashion, and the urge to remove too thick a section must be resisted. In celloidin sectioning, patience is an absolute necessity.
References
- Bolles-Lee, A. (1913)
The Microtomist’s Vade-Mecum Ed. 7.
P. Blakiston, Philadelphia, USA






