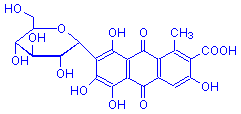
Cochineal Carminic acid
Description
Carminic acid is the active colouring agent in both carmine and the less pure cochineal. It is rarely used in practice, however, since the considerably more easily obtained carmine is as effective in biological staining. Carmine is a valuable dye, which is invariably applied in conjunction with a mordant, usually aluminum. It can be used to stain glycogen (Best's carmine), acid mucopolysaccharides (mucicarmine), and nuclei (carmalum). Carmine is obtained from the bodies of the female of the insect Dactylopius coccus, also known as Coccus cacti.
Insect Dyes
References
- R. D. Lillie.
Conn’s Biological Stains
Williams & Wilkins, Baltimore, MD., U.S.A. - Susan Budavari, Editor,
The Merck Index, Ed. 12
Merck & Co., Inc., Whitehouse Station, NJ, USA - Aldrich chemical catalogue, 1992
Aldrich Chemical Company, Milwaukee, WI, USA.






