In histotechnology, the word lipid refers to all fat and fat-like, or fat-containing substances. This includes triglycerides, fatty acids, lipoproteins and glycolipids. It would also include any material that has a fat or fatty material as a component, regardless of the amount. In other words, it is an all-encompassing, general term with little specificity. When a more specific reference for these materials is required, it is usual to use a term describing the particular kind of lipid under discussion. The term fat is often used in the same fashion as “lipid”.
There is a difficulty with demonstrating lipids histologically. It is that they dissolve in the solvents used for paraffin processing and, to a lesser degree, in celloidin processing. That means they may not be present in paraffin sections, having dissolved out. For that reason, frozen sections may be required. It should be noted, however, that not all lipids are removed by the solvents used during paraffin processing and significant amounts of some lipids may still be present. Generally, these are lipids bound to other materials, such as lipoproteins, lipofuscins and myelin. Triglycerides are always completely removed in properly cleared and infiltrated blocks, and the statement that fats are removed by paraffin processing is a reference mainly to this.
A large amount of lipoprotein is present in cell membranes and, although this is not removed by paraffin solvents, it is not usually demonstrable with normal methods for lipids. It is often referred to using the non-specific term of masked lipids, lumping it together with other, unspecified, materials.
Triglycerides
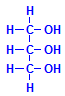
Ordinary fats are esters of glycerol and fatty acids. Glycerol is shown to the left. It can combine with three molecules of fatty acids, whether all the same or different. The resulting compounds are the triglycerides and are very common. They are usually what is meant when otherwise unqualified references are made to fats. Oils are much the same, the difference being that fats are solid at room temperature, while oils are liquids. It should be noted that in this context the word “oil” refers to vegetable and animal oils. It does not refer to the material pumped from the ground. As well as triglycerides, glycerol can form monoglycerides with one fatty acid and diglycerides with two.
The second component of triglycerides is the fatty acids. These are chains of carbon atoms linked together and terminating with a carboxyl group. The simplest is acetic acid, but those involved in the formation of triglycerides have many more carbons than acetic acid. Palmitic acid has 16 carbons, while stearic acid has 18, as does oleic acid. All three are common fatty acids in triglycerides. The difference between stearic acid and oleic acid, both of which have carbon chain lengths of 18, is that stearic acid is saturated while oleic acid is unsaturated. In other words, the saturated stearic acid has as many hydrogen atoms attached to its carbon atoms as is possible, while the unsaturated oleic acid has less than the maximum number and the carbon atoms involved have an extra bond to each other. The bottom right formula of those just below shows this explicitly and the one to its left shows it how it is normally represented. Oleic acid has only one double bond but other fatty acids may have more than one. Liquid oils tend to have more unsaturated fatty acids than solid fats.
Palmitic acid
Stearic acid
Oleic acid
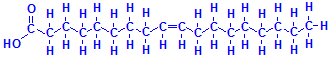
Oleic acid, explicit
A triglyceride is shown below using the three fatty acids whose formulas are given above. It should be kept in mind that there are many more fatty acids than these three and they can form triglycerides in many combinations, making the number of possible compounds very large. They may exist as mixtures, but histologically they are not individually demonstrable. These compounds are the “fat” that fat stains such as oil red O demonstrate by dissolving in them.
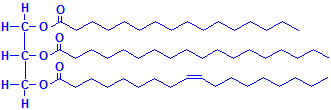
Triglyceride
Although it is not possible to identify individual fatty acids, it is possible to demonstrate free fatty acids as a group. The methods depends on the reaction between a copper salt with the fatty acid, then staining the copper to give the location of the fatty acid, or on the selective staining of fatty acids in a contrasting color to triglycerides using a solution of the dye Nile blue, which has a significant amount of the degradation product Nile red. The methods do not have the reputation of being thoroughly convincing.
Phospholipids
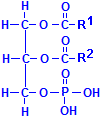
Fatty acids are not the only acids that may combine with glycerol. Phosphate is very common as well. If one of the fatty acids is replaced with phosphoric acid, the resulting compounds are collectively called phospholipids. In the illustration to the left of this paragraph, R1 and R2 refer to fatty acids. In addition, other groups may be attached to the phosphate, phosphatydilcholine, a major constituent of lecithin, for instance, has choline attached to the phosphate. There are similar compounds with inositol, serine, ethanolamine, etc.
These compounds are extensively found in tissues as a component of various membranes as they are hydrophobic at one end of their molecule and hydrophilic at the other. When oriented all in the same direction, membranes incorporating them can form a barrier that permits water soluble materials to approach on one side but keeps water soluble constituents from passing through the barrier from the other. Phosphatydilcholine is shown below. Other phospholipids have similar formulas, but with the choline shown on the bottom right, –(CH2)2N(CH3)3, replaced by the appropriate group.
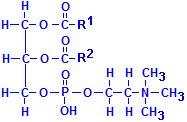
Phosphatydilcholine
Sphingolipids
Glycerol is not the only material that can combine with fatty acids to form lipids. Sphyngosin can also do so producing sphingolipids, which are major components of myelin. A fatty acid can attach to the amino group of sphyngosin, producing a ceramide, the second formula below illustrated with palmitic acid. Other groups may be attached as well, including phosphate, and to the phosphate groups other groups such as choline can attach, much as with phospholipids. These compounds are collectively referred to as sphingomyelin, which is the third formula below, illustrated with phosphate and choline. If instead of phosphate and choline a monosaccharide is attached, the resulting compound is referred to as a cerebroside, illustrated as the fourth formula below. If the sugar attached is an oligosaccharide, the resulting compound is called a ganglioside. It differs from the cerebroside by the number of sugar molecules attached. There is an illustration of a ganglioside on Wikimedia Commons. Both cerebrosides and gangliosides are also classed as glycolipids. The sugars in these compounds may also be sulphated, in which case they are called sulphatides, illustrated as the fifth diagram below. Wikimedia Commons has an illustration of a sulphatide as well.

Sphingosine

Ceramide
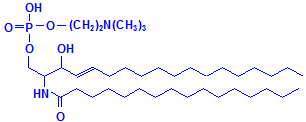
Sphingomyelin
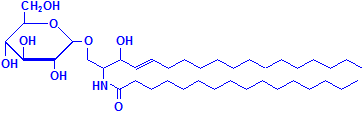
Cerebroside
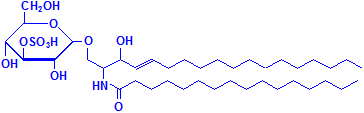
Sulphatide
The diversity among the sphingolipids and the phospholipids explains the wide range of staining methods that can demonstrate myelin, of which they are major components. The sugars of cerebrosides and gangliosides may be oxidizable to aldehydes and stained with Schiff’s reagent. The phosphate may take part in dye staining, and may facilitate mordant dyeing, which is so commonly used to demonstrate myelin with iron hematoxylin. Similarly, components of the choline, ethanolamine and like compounds, may be involved in dye staining, as may the sulphate of sulphatides. In addition, the fundamental structure is that of either fatty acids bonded to glycerol, or fatty acids bonded to sphingosine, which is itself a long chain hydrocarbon. These factors enable solvent dyeing with dyes such as oil red O and sudan black B. Sphingosine also has hydroxyl and amino groups attached that may be capable of participating in dye binding. Unfortunately, with all the possibilities, it is difficult to know precisely what does happen.
Lipoprotein
Lipid-protein complexes are more of an anomalous group. Any lipid bound in any way to a protein is usually referred to as a lipoprotein histologically. Unfortunately, a major part of the lipoprotein in tissue cannot be demonstrated. The lipids found in cell membranes of various kinds are usually bound to protein, so much so that the lipid component cannot be stained separately by the usual staining methods. In many cases, lipoproteins function to transport lipids around the body, so may not be localized to particular tissue components. Those that are may be demonstrated through their fat content. One such is lipofuscin, or “wear and tear” pigment. When first formed it has a higher lipid content than after it has existed for some time, and for that reason, staining results may vary.
One characteristic of these fatty compounds is that they are variably resistant to extraction by processing solvents. Xylene will extract all triglyceride in the time usually allowed for paraffin processing, but part of the lipid found in myelin and lipofuscin may not be removed and may still give some light staining. Those most resistant are likely to be those with significant non-lipid components and that are bound to tissue proteins. Certainly, sufficient remains to demonstrate myelin in paraffin sections quite well, and a standard method for lipofuscin is an oil red O stain on a paraffin section.






