An accentuator is any chemical which facilitates the staining process. Usually the purpose is to intensify staining, and accentuation with this meaning is obviously the derivation of the term. However, it should be noted that inhibition of staining can also accentuate a structure in comparison to the background staining. Inhibition and accentuation of staining are just two sides of a coin, and will be discussed together.
Accentuators fall into three groups: pH control, salts and phenol.
pH Control
Staining depends largely on the attachment of dyes to proteins. These have both positively and negatively charged groups. In proteins, the concern is with positively charged amino groups, and negatively charged carboxyl and hydroxyl groups. In addition, phophate groups of DNA are important in nuclear staining. Dyes also have the same groups as the proteins, but may include the sulphonic group as well. Which of these groups is involved in any particular case depends on the circumstances, including the pH of the staining solution.
Although proteins have both positively and negatively charged groups, usually one predominates and they will have an overall negative or an overall positive charge (be an acid or a basic protein). These charges can, however, balance each other out to some degree. At a particular pH, each protein will have an overall charge of zero. This is different for each protein, and is its isoelectric point.
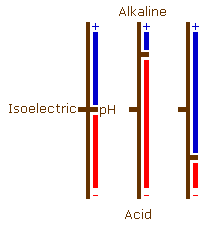
If the pH is raised above the isoelectric point, then the number of charged amino groups is reduced and the number of charged carboxyl and hydroxyl groups increases causing it to behave as an acid protein. As the pH is lowered from the isoelectric point the number of charged amino groups will increase and the number of charged carboxyl and hydroxyl groups will decrease causing the protein to behave as a basic protein.
If these pH changes are carried to an extreme the situation arises where (at acid pH levels) there are no charged carboxyl or hydroxyl groups, and (at alkaline pH levels) there are no charged amino groups.
There are four possibilities with regard to minor pH adjustment, two accentuate and two inhibit staining.
| Dye | Solution Added | Effect |
|---|---|---|
| Acid dye | Acid added | Increased staining |
| Acid dye | Base added | Decreased staining |
| Basic dye | Acid added | Decreased staining |
| Basic dye | Base added | Increased staining |
In most cases, pH adjustment is relatively minor. Acidification of solutions is usually done with acetic acid in 1% – 2% concentration. The final pH is usually about 4, depending on the buffering capacity of other ingredients. This increases the ionisation of tissue amino groups. Similarly, minor adjustments to make solutions more alkaline can be done with compounds such as sodium tetraborate (borax) or sodium carbonate. At a pH of about 8, these increase the ionisation of carboxyl groups.
Examples of pH adjustment to increase staining intensity is found in the use of acetic acid in conjunction with acid dyes in Masson’s trichrome, and the addition of borax to methylene blue. Both increase the intensity of staining.
An example of pH adjustment to inhibit staining is found in the practice of adding a very small amount of acetic acid to 1% aqueous solutions of neutral red in order to sharpen nuclear staining. This small amount of acid slightly inhibits the ionic staining of background tissues, making the largely unaffected ionic nuclear staining appear more prominent.
If the pH is adjusted outside the range of about 4 – 8, then some groups cease to ionise altogether, and their staining is inhibited almost completely. At a pH of about 10 amino groups are unavailable. For carboxyl groups the relevant pH is about 4 and below. These pH numbers are not absolutes. As the pH alters, there is an impact on staining, but the impact is gradual. It should also be noted that the dyes we use are effected by the pH changes, but are present in such large amounts in comparison to the tissue groups involved, that the effect on them can be largely discounted.
Certainly, when the pH is at 1.5 no carboxyl groups are involved with staining. However, phosphate radicals are still ionisable at that pH and nuclear staining can still be done, although it tends to be highly selective. To see this, replace the citric acid in Mayer’s hemalum with 1 mL concentrated hydrochloric acid. Compare the staining.
It is also used to good effect in the demonstration of amyloid with congo red and similar acid dyes. Sodium hydroxide (or a buffer) is added to the staining solution. The pH of this kind of solution is above 10, and has the effect of inhibiting ionic staining as much as possible. As a consequence, the significantly lighter staining due to hydrogen bonding becomes more prominent instead of being overshadowed by the more intense ionic staining.
Salts
The value of chemical salts lies in their being ionic compounds. When dissolved, they will produce in solution both negatively and positively charged ions. We are not here talking about those salts which are used as mordants, but rather what are sometimes called indifferent salts. These are compounds, such as sodium chloride, which appear to have no part to play in the staining process.
When a solution with an indifferent salt in conjunction with a dye is applied to tissue, the negatively charged ions in solution are attracted to positively charged tissue groups. Similarly, positively charged ions are attracted towards negatively charged tissue groups. Usually this would be pointless, as we would want a positively charged dye ion to be attracted towards a negatively charged tissue component, and bond to it.
Sometimes, however, non-ionic bonding is the goal, and we might want to demonstrate a negatively charged material with a negatively charged dye. The presence of positively charged ions from an indifferent salt can satisfy the ionic attraction of the negatively charged tissue group, and in the process the ionic repulsion that two negatively charged ions exhibit will be eliminated. The result is that an acid dye can approach an acid material closely enough that hydrogen bonding or van der Waal’s forces can bind them.
The scenario above is not, in fact, hypothetical. It is the explanation for the inclusion of sodium chloride into ethanol solutions of congo red for the demonstration of amyloid. Its inclusion enables a higher degree of hydrogen bonding than would otherwise be the case. The salt is therefore an accentuator in the sense of increased staining.
There is a similar application in the Critical electrolyte concentration (CEC) method for differentiating acid mucopolysaccharides. Mucopolysaccharides are ionic complexes and react with alcian blue ionically. The addition of magnesium as an ionic salt preferentially inhibits staining with the dye by competing with it for the negative charge on the mucosubstance. Since different acid mucopolysaccharides require different concentrations of magnesium ions to overcome the attraction between alcian blue and the mucosubstance, it can be used to differentiate between them.
Phenol
How phenol accentuates staining is still not completely clear. The explanations tend to be speculative, and are not particularly convincing.
It has been suggested that the mild acidity produced by solutions of phenol is the cause of intensified staining, and adding phenol is fundamentally pH control. If this were the case, the expected staining pattern would be that acid dyes would stain more intensely, and basic dyes would stain less intensely, since that is what adding acid to staining solutions usually accomplishes. In fact, the commonest use of phenol accentuation is in carbol fuchsin, which uses a basic dye. It also intensifies acid dye solutions, seen with carbol chromotrope. Since both basic dyes and acid dyes can be intensified with phenol, it is very unlikely that its mild acidity is the cause.
The second explanation depends on the masked lipid limitation on aqueous staining. This explanation says that lipids associated with protein inhibit the approach of watery solutions as they are mildly hydrophobic. This reduces the completeness of staining from aqueous solution. Phenol lowers the surface tension of aqueous solutions, enabling the water to wet the tissue more thoroughly. This, in turn, enables the dissolved dye to more thoroughly contact the proteins and bond to the available ionised groups.
A variation of the second explanation is usally given as to why carbol fuchsin demonstrates acid fast organisms. Mycobacteria contain fatty acids (mycolic acids) in their cell walls and are hydrophobic. The hydrophobic effect is overcome with phenol as it lowers the surface tension allowing the lipids to be wetted by the carbol fuchsin, but at room temperature the dye is unable still to penetrate the fatty acid. When heated, the fatty acid softens and the dye enters the cell. When it cools the dye is trapped inside the organism and the acid alcohol can’t remove it.
It has also been explained on the basis of the phenol enabling the dye to actually dissolve into the mycolic acids. The rationale is that the phenol competes with the dye anion (acetate ion) and liberates the cation (basic fuchsin free base). It is then to be understood that this is soluble in lipids, and when the lipid is melted by the gentle heat applied, the free dye base dissolves into it. When the lipid cools, the dye is not able to be extracted. The difficulty with this explanation is that we would normally free a cation (dye base) by adding a competing cation to preferentially combine with the anion (acetate). Phenol would not normally combine with acetate, although it could replace it, forming basic fuchsin phenolate. The phenolate could then act as a carrier for the dye. This explanation is very speculative, and consequently unreliable, as is plain.
References
- R. D. Lillie.
Conn’s Biological Stains
Williams & Wilkins, Baltimore, MD., U.S.A. - Baker, John R., (1958)
Principles of biological microtechnique
Methuen, London, UK.






