Schiff’s reagent is undoubtedly the most common way of demonstrating the presence of aldehydes in tissues, but it is not the only possibility. The presence of aldehydes may also be shown with a methenamine silver reduction, a diazonium coupling reaction between 2-hydroxy-3-naphthoic acid hydrazide and fast blue B, an osmium reduction following thiosemicarbazide treatment, and a sequence using thiosemicarbazide followed by Schmorl’s reaction. Of these, the silver reduction is quite popular for the demonstration of fungi and basement membranes. The others are not common at all.
Considerations
With all of these methods, of course, aldehydes must first be produced in the sections. This can be done by all the usual techniques such as periodic acid or chromic acid oxidation, by acid hydrolysis of DNA as in a Feulgen reaction, or by techniques which depend on the attachment of aldehydes to tissues groups for histochemical use.
All of these other techniques are either improved by, or depend on, the use of hydrazine based compounds. Most commonly this is thiosemicarbazide, but one of them (naphthoic acid hydrazide) has hydrazine attached directly to the naphthoic acid involved. The reason is that hydrazine based compounds can react with aldehydes allowing for other chemical groups to be attached to the aldehydes, and through which they may be demonstrated.
Silver Reduction
Methenamine silver reduction is an exception, in that a hydrazide is not an integral component of the technique. The aldehydes themselves can reduce methenamine and ammoniacal silver solutions directly, usually at elevated temperatures of 60°C or so. This is the basis of the Grocott and Jones methods for fungi and basement membranes respectively. Despite this, thiosemicarbazide may be used to increase the speed and efficiency of the reduction, improving the depth and contrast of the impregnation.
Osmium Reduction
Osmium reduction does require a hydrazide, however, and is exemplified in the periodic acid thiosemicarbazide osmium (PATO) technique for carbohydrates. This is effective for both light and electron microscopy, although possibly more common in the latter. It is not popular in light microscopy since it requires osmium tetroxide, a compound which is very expensive, can cause health problems, must be used under a fume hood, and requires disposal as a toxic heavy metal.
Hydrazine Reactions
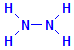
The remaining ways of demonstrating aldehydes depend on the use of hydrazine. As you can see from the formula to the left, it is fundamentally two amino groups attached to each other through their nitrogen atoms.
Its usefulness in the context of aldehyde demonstration is twofold. One is that it can replace amino groups in other compounds, forming hydrazides. The other is that these hydrazides may be able to react with aldehydes through the hydrazine group, but at the same time the chemical to which the hydrazine is attached may still remain active and continue to be able to participate in reactions with other reagents. In this way the hydrazine acts like a carrier which can be used to attach reactive groups to aldehydes and, through those groups, demonstrate the aldehyde’s presence.
Azo-Coupling
Azo coupling of a diazonium salt to a naphthol is a common method of manufacturing azo dyes. It is also a common feature of many histochemical techniques for the demonstration of enzymes. It is also possible for this mechanism to be used to demonstrate aldehydes. This ability of hydrazides to carry a reactive group and attach it to aldehydes is illustrated in the coupling azo dye technique. This method depends on the attachment of 2-hydroxy-3-naphthoic acid hydrazide (also known as 3-hydroxy-2-naphthoic acid hydrazide) to aldehydes, then demonstrates the naphthoic acid component with a diazonium salt (fast blue B).
2-hydroxy-3-naphthoic acid hydrazide is illustrated below, with the hydrazine group shown in red. This hydrazine reacts with tissue aldehydes, attaching the naphthoic acid to the tissue.
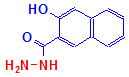
Naphthoic acid hydrazide
+
Aldehyde
=
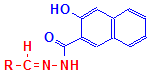
Product
+ H2O
Following formation of the naphthoic acid-aldehyde complex, a diazonium salt is applied. This reacts with the naphthoic acid to form a blue dye. The aldehydes therefore appear blue in a finished section. This is illustrated below, but please note that the reaction has not been balanced. The final product also shows the diazonium salt attached to two aldehyde groups. This may not be the case, but is shown for convenience to illustrate that the final product is an azo dye attached to the aldehyde groups originally produced in the tissue. It is likely, also, that other diazonium salts could be used, producing a range of colors.

Naphthoic acid-aldehyde product
+
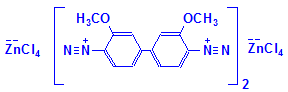
Fast blue salt B
=
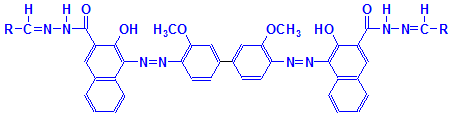
Final Product
As you can see, the final product has a structure typical of a disazo dye. This final product is reproduced below with each of the components coloured separately to show the reactions involved more clearly.
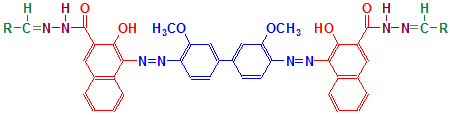
- Green – Aldehyde
- Purple – Hydrazine
- Red – Naphthoic acid
- Blue – Diazonium salt
Thiosemicarbazide Reactions
The other techniques use thiosemicarbazide to facilitate the demonstration of aldehydes. The explanation is very similar for all of them, being based on the the fact that thiosemicarbazide has both a hydrazine group that will react with aldehydes and a thiocarbamyl group that is a strong reducing group, more so than are tissue aldehydes, and that this thiocarbamyl group remains active after the thiosemicarbazide has attached to tissue aldehydes. It is also the explanation for why silver reductions are improved when this reagent is used after aldehydes have been formed in Grocott’s and Jones techniques.
Since the common name for this compound is thiosemicarbazide it is sometimes assumed that it is somehow related to metallic azides, compounds which are known to be explosion hazards. This is not the case, and thiosemicarbazide is safe to use. It is, in fact, more closely related to urea than to metal azides. The first point to make clear is that it is not an “azide”. but a “carbazide”. Azides have nitrogen atoms bonded together, whereas semicarbazide has a nitrogen bonded to a carbon.
In the formulas below compare the formula for urea with the formula for semicarbazide. The relevant parts are coloured in red. Then compare the semicarbazide with the azide. It is quite clear that they are not closely related structures at all and have no similarity other than the presence of nitrogen atoms. Semicarbazide is simply urea which has had one of its amino groups replaced with hydrazine. If both amino groups had been replaced it would be known as carbazide.
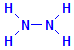
Hydrazine
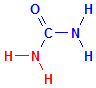
Urea
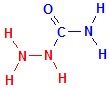
Semicarbazide
Azide
Thiosemicarbazide is simply semicarbazide in which the oxygen bonded to the carbon atom has been replaced with a sulphur atom. This means that thiosemicarbazide is composed of a hydrazine group bonded to a thiocarbamyl group. The latter is a strong reducing group. Thiosemicarbazide is also known as N-aminothiourea and hydrazinecarbothioamide, names which imply no relationship with metallic azides.
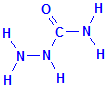
Semicarbazide
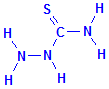
Thiosemicarbazide
Various MSDS on this chemical make it clear that there is no explosion nor fire hazard. Please see the Baker, the Sigma-Aldrich and the Anachemia documents as typical examples. It is, however, poisonous and may form toxic gases when in a fire. In practice, it appears to be no more dangerous than many of the reagents we use, and normal safe working practices are all that is required.
The reaction with aldehydes is illustrated below, with the relevant components coloured in red. Compare this to the first set of illustrations above on the reaction between 2-hydroxy-3-naphthoic acid hydrazide and aldehydes. Apart from the molecule being carried, the reaction is the same, emphasizing that hydrazine can be used to attach reactive groups to aldehydes. In the case of thiosemicarbazide, the reactive group added is a thiocarbamyl group which is a fairly strong reducing group, stronger than the original aldehyde. This enables techniques which utilize the reducing capability of aldehydes to continue to be used and invariably gives faster and stronger reductions. This means that demonstrations of the original material are improved, whether it be with a methenamine or ammoniacal silver reduction, osmium reduction, or Schmorl’s ferricyanide reduction.
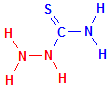
Thiosemicarbazide
+
Aldehyde
=
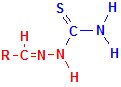
Product
+ H2O






