Schiff’s reagent has been used to demonstrate proteins following treatment with various agents. Usually the group targeted is the amino and similar groups. The methods are of two types: reactions with aldehydes, and oxidative deamination producing aldehydes. These techniques are not commonly used in service laboratories and remain more useful in investigative settings. They are included here to show the full range of applications which employ Schiff’s reagent.
Reaction with Aldehydes
Aldehydes can react with tissue groups. That is, after all, the underlying principle of formaldehyde fixation. The question arises, therefore, as to whether these aldehydes can be attached to tissues and then be demonstrated with Schiff’s reagent. In the case of formalin the answer would appear to be no.
However, glutaraldehyde (glutaric dialdehyde) is a fixative which leaves tissue with reactive aldehyde groups. If Schiff’s reagent is applied a positive reaction is obtained without prior oxidation. This is a known problem with glutaraldehyde fixation because it interferes with the PAS reaction. In order for a PAS reaction to be valid with this fixative, it is first necessary to eliminate these aldehydes. Other than that problem, glutaraldehyde has not been used in Schiff type procedures
A second aldehyde, acrolein (acrylic aldehyde), has been used in a more systematic manner. It forms compounds with sulphydril, amino, and imidazole groups which leave its aldehyde group free to react with Schiff’s reagent. It is consequently suitable for the specific demonstration of proteins. It is not in common use.
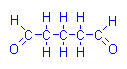
Glutaraldehyde
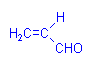
Acrolein
Oxidative Deamination
Ninhydrin, alloxan and chloramine T have all been used in methods depending on oxidative deamination of amino acids. That is, oxidizing agents which remove amino groups and produce a tissue aldehyde in the process which can then react with Schiff’s reagent. Of these three the ninhydrin-Schiff reaction has gained some favour for the demonstration of proteins containing lysine, hydroxylysine, glutamine and aspartine. It produces a blue colour with these amino acids and forms an aldehyde which can react with Schiff’s reagent. The alloxan-Schiff reaction is similar, and has been used in a method for demonstrating rabies virus, but chloramine T is rarely employed.
Ninhydrin-Schiff Reaction
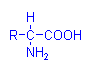
Amino Acid
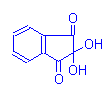
Ninhydrin
⇒
Aldehyde
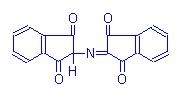
Blue Product
Alloxan-Schiff Reaction
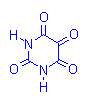
Alloxan
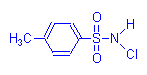
Chloramine T
⇒
Aldehyde

Blue Product
References
- Lillie, R.D., (1954)
Histopathologic technique and practical histochemistry Ed.2
Blakiston, New York, USA. - Pearse, A. G. E., (1968, 1972)
Histochemistry: Theoretical and Applied, Ed. 3
Churchill Livingstone, Edinburgh, London, UK - Aldrich chemical catalogue, 2003-4
Aldrich Chemical Company, Milwaukee, WI, USA. - Susan Budavari, Editor, (1996)
The Merck Index, Ed. 12
Merck & Co., Inc., Whitehouse Station, NJ, USA - Chloramine T
Upper Midwest Environmental Sciences Center, Wisconsin, USA