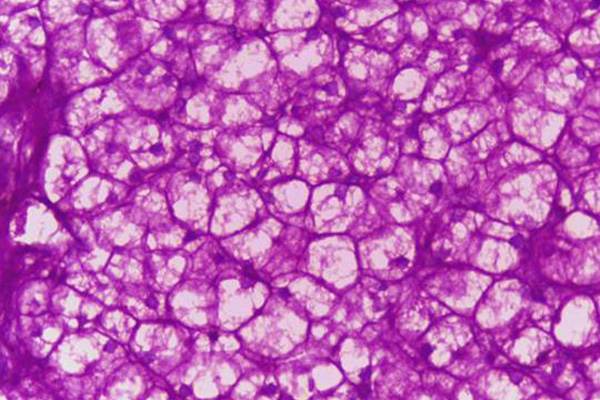
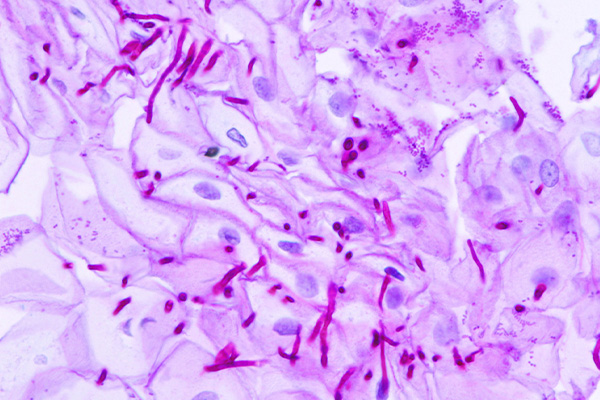
Likely the most common application for Schiff’s reagent is the Periodic Acid Schiff, or PAS, reaction. This is a technique for the demonstration of carbohydrates in tissue sections. The purpose of the periodic acid is to oxidize some of the tissue carbohydrates. This produces aldehyde groups, which can then condense with Schiff’s reagent forming a bright red coloration and demonstrating the tissue component to which the carbohydrate is attached.
Introduction
The PAS reaction is quite straightforward. Sections are first brought to water, then a 1% aqueous solution of periodic acid is applied for 10 to 30 minutes. The sections are washed to remove any traces of the periodic acid, then Schiff’s reagent is applied for a while, again for 10 to 30 minutes. The Schiff’s reagent is rinsed off with water, then the sections are washed with water until the water is clear and the sections look pink, usually about 10 to 30 minutes. The color deepens somewhat as the washing continues, within limits. The time for which the reagents are applied can affect the intensity of the color. Also, some substances are known to require extended oxidation, and may not be oxidized within the times given.
The treatment with periodic acid oxidizes some of the carbohydrates in the tissue to aldehydes. The carbohydrates involved are 1-2 glycols, shown below in red. Since not all carbohydrates include this structure, the PAS is not a method for carbohydrates in general but only for those which contain 1-2 glycols or closely related structures, such as one in which an amino group replaces one of the hydroxyl groups, also shown below in red. Carbohydrates that stain with this method include polysaccharides, mucopolysaccharides, glycoproteins, and glycolipids. The essential point is that treatment must be able to produce an aldehyde on the carbohydrate component.
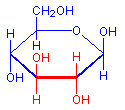
1-2-glycol
+ HIO4
=
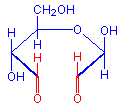
Aldehyde
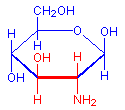
1-amino-2-hydroxy
+ HIO4 =

Aldehyde
Staining Criteria
According to Culling, Hotchkiss stated that the following criteria were necessary for a tissue substance to give a positive PAS reaction:
- It must contain the 1-2 glycol grouping, an equivalent amino or alkylamino grouping, or the oxidation product CHOH-CO.
- It must not diffuse away during fixation.
- It must give an oxidation product that does not diffuse away.
- It must be present in sufficient concentration to produce a detectable final color.
The following list, also based on Culling, gives the PAS-positive materials usually encountered, although it is not exhaustive:
- Actinomyces clubs
- Basement membranes
- Cartilage
- Cerebrosides
- Collagen from areolar connective tissue
- Compound lipids
- Glycogen
- Kerasin (Gaucher disease)
- Kidney tubules
- Megakaryocyte granules
- Ocular lens capsule
- Paneth cell granules (some species)
- Pituitary beta cells
- Retinal rods
- Starch
- Mucins of:
- Intestinal tract
- Peptic gland
- Uterine cervical glands
- Salivary glands
- Conjunctiva
- Bronchial glands
- Ovarian follicles and cysts
- Prostatic gland secretion
- Corpora amylacea
- Pancreatic zymogen granules
- Phospholipids
- Renal hyaline casts
- Russell bodies
- Thyroid colloid
Periodic Acid Solutions
The following formulae for periodic acid solutions in the PAS reaction are given by Lillie. He comments that it seems to make no difference which aqueous solution is used as a routine. It should be noted that the 90% ethanol variant takes a much longer time than the others. However, it is reputed to demonstrate all oxidizable material.
Common preference today is for a simple 0.5% or 1% aqueous solution, although a 0.1% solution is satisfactory. The solution is usually applied for about 10 minutes, even though some early recommendations were for 2-3 minutes. Within limits, the longer the periodic acid as applied the darker the staining for the same time in Schiff’s reagent. Periodic acid solutions may be re-used.
In the chart below, the amount of oxidant given is intended to be dissolved in 100 milliliters of the solvent. All of the solutions are stable.
| Variant | Oxidant | Amount | Solvent | Time |
|---|---|---|---|---|
| McManus | Periodic acid | 0.5 g | Distilled water | 5 minutes |
| Lillie A | Sodium iodate | 1 g | Nitric acid, 0.5% aqueous | 10 minutes |
| Lillie B | Potassium iodate | 0.8 g | Nitric acid, 0.3% aqueous | 10 minutes |
| Lillie C | Potassium iodate | 0.69 g | Nitric acid, 0.3% aqueous | 10 minutes |
| Hotchkiss A | Periodic acid | 0.8 g | Sodium acetate, 0.02M aqueous | 5 minutes |
| Hotchkiss B | Periodic acid | 0.8 g | Sodium acetate, 0.02M in 70 ethanol | 5 minutes |
| Mowry | Periodic acid | 1 g | 90% ethanol | 120 minutes |
Other Oxidizing Agents
Periodic acid is not the only oxidizing agent that has been recommended for the oxidation of carbohydrates in this kind of reaction, although it is certainly the most used and arguably the most effective. Chromic acid (chromium trioxide in water) is often used and forms the basis of a method for fungi. It is different from periodic acid in that it continues to oxidize the aldehydes it produces. Over-oxidation will therefore eventually lead to a pale or false negative reaction. Some other oxidizing agents have been used as well including permanganic acid, performic acid, peracetic acid, and lead tetraacetate. Although valuable for specific purposes, these are not in common use.
Double Oxidation
Periodic acid oxidation of carbohydrates to aldehydes and the subsequent demonstration of the aldehydes are important for the staining of 1-2-glycols and the histological demonstration of many structures. Periodic acid, however, has been less popular for fungal cell walls as staining carbohydrates in the background may diminish the contrast between the background pink and the deeper pink of the fungal cell wall. To overcome this, the oxidant used for fungi is often chromic acid. This reagent has become popular due to its characteristic of continuing to oxidize the aldehydes initially produced to carboxylic acids, which no longer take part in staining. This allows for a reduction in background intensity, as much of the easily oxidized material present in it no longer stains. As a consequence, the fungi, and other structures, may appear more prominent in contrast.
The double oxidation PAS accomplishes much the same but permits more control over the amount of carbohydrate that is inhibited from staining. The aldehydes produced by the first oxidation are blocked by the aniline-acetic aldehyde block. Changing the duration of the periodic acid in the first oxidation will allow for more or less aldehyde to be produced, depending on the time it is applied. Regardless of the amount produced in this first oxidation, all of it will be inhibited from staining with Schiff’s reagent and it will not be seen. At the same time, any pre-existing aldehydes present, from glutaraldehyde fixation, for instance, will also be blocked and will not be seen in the finished section either.
The second oxidation then produces more aldehyde from the remaining, previously unoxidized, carbohydrate. Once again, the amount of aldehyde produced, and the intensity of the staining of the associated carbohydrates depends partly on how long the second application of periodic acid is. There is a limit, of course, which is determined by the absolute amount of oxidizable carbohydrate remaining after the blocking step. Once this carbohydrate has all been oxidized, no more aldehyde will be produced, but neither will extend oxidation with the periodic acid diminish the amount of aldehyde produced.
Aldehyde Blocks
Aldehyde blocks to prevent the reactions of aldehydes may be applied either before or after periodic acid oxidation, depending on which aldehydes are the target. To block pre-existing aldehydes, the block may be applied before periodic acid oxidation. To block aldehydes formed during periodic acid oxidation, the block may be applied after this step. For additional details on blocking solutions and considerations for use, consult the guide below.
Adjusting the Intensity of Staining
The original recommendations for a standard PAS were that both oxidations in 1% periodic acid and treatment with Schiff’s reagent should be for about 10 minutes each. This gives distinct, but not especially intense, staining. Some microscopists prefer a more vibrant depth of red coloration, particularly when it is for histological demonstration of structures rather than a histochemical evaluation of carbohydrate content. The intensity of staining depends on three factors:
- The duration of oxidation and the amount of aldehyde actually produced,
- The length of time in Schiff’s reagent,
- The quality of Schiff’s reagent.
Increasing the oxidation time will usually increase the depth of staining. However, if the amount of target material is sparse, it may all be oxidized fairly quickly and further oxidation will have no effect on it, although it may increase oxidation of other materials so they stain more intensely and mask a paler stained target material.
Longer applications of Schiff’s reagent also usually increase the depth of staining. Again, this can be overdone, and very intense staining may mask some more weakly stained material.
A good quality Schiff’s reagent is essential. This means that it should be made from pararosaniline rather than basic fuchsin, i.e. the specific component of basic fuchsin which produces the brightest staining solution. Beware of dye batches that give a brown solution when first made. Only a water-clear or pale amber solution is acceptable. Although this brown discoloration may be removed with activated charcoal, the original depth of brown is indicative of the amount of non-pararosaniline components present in the dye. If large amounts of charcoal are needed to remove the brown color, or the charcoal has to be allowed to stay in the solution for longer than about a minute to make it water clear, then it is quite possible that the staining abilities of Schiff’s reagent may be compromised. Purchased Schiff’s reagent is usually satisfactory. In any case, all new batches of Schiff’s reagent should be tested against a known tissue with which you are familiar, to ensure that batch-to-batch staining is the same or very similar. The same tissue should be used as a staining control whenever a PAS is done.
References
- Kiernan. J.A., (1999)
Histological and histochemical methods: Theory and Practice, Ed. 3
Butterworth Heinemann, Oxford, UK. - Culling C.F.A., (1963)
Handbook of histopathological techniques Ed. 2
Butterworth, London, UK. - Culling C.F.A., (1974)
Handbook of histopathological and histochemical techniques Ed. 3
Butterworth, London, UK. - Pearse, A. G. E., (1968, 1972)
Histochemistry: Theoretical and Applied, Ed. 3
Churchill Livingstone, Edinburgh, London, UK - Lillie, R.D., (1954)
Histopathologic technique and practical histochemistry Ed.2
Blakiston, New York, USA.