Hematoxylin itself is not a dye, and it has to be oxidized to hematein, which is a dye before it can be used. This process is called ripening and can be accomplished in two distinct ways. Simple alcoholic or aqueous solutions made with hematoxylin are usually pale yellow-brown in color. On oxidation the color changes to a deep, mahogany brown. When combined with an aluminum salt such as aluminum potassium sulphate, the colors are pale, transparent violet (unripened), and deep opaque purple (ripened). They may also be combined with iron salts. In these, the color is deeper, usually a very dark violet.
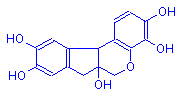
Hematoxylin
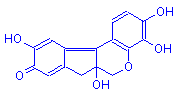
Hematein
Natural Ripening
It was common practice in the past to use natural oxidation in the belief that it gave a more reliable and longer lasting solution. Natural ripening is accomplished by putting the solution in an oversize flask, so it can be shaken, with the top plugged loosely with cotton wool, allowing air to enter. This is left in a warm, light, and airy place (a windowsill) for oxidation to take place. The flask is shaken periodically. Oxidation may take several months and is determined by testing the solution from time to time. When the solution gives a satisfactory depth of staining, it is transferred to a brown bottle and tightly stoppered for storage in the dark to retard further oxidation.
Natural Ripening with Ethanol
If naturally ripened alum hematoxylin solutions are wanted, but the time for ripening is too inconvenient, then a strong alcoholic solution might be the answer. Hematoxylin saturates in ethanol at about 3% and Hematein does so at about 7%.
- Place more than 7 grams hematoxylin in 100 mL of absolute ethanol. Adjust the amount for the actual dye content of the sample and allow a little more to ensure saturation.
- Store in a light, airy place and agitate periodically until it turns a deep, rich brown.
- Do NOT filter out undissolved dye. The excess ensures saturation (about 7%).
- Stopper tightly and store in the dark.
- Use 14 mL of this solution for each gram of dye required.
- Solutions made from it should be usable immediately.
- Although these saturated ethanolic solutions last for years, they do eventually deteriorate due to continued oxidation.
In practice, experiences from very large numbers of histotechnologists have shown that the most convenient and reliable approach is to start with hematoxylin and oxidize it to hematein, either completely or partially. With sodium iodate, this can be so fast and easy that hematein is scarcely needed at all.
Chemical Ripening
The other way to ripen hematoxylin is to use chemical oxidizing agents. The most commonly used is sodium iodate, at about 200 mg for each gram of hematoxylin. Others have also been suggested for particular formulas, but sodium iodate can be substituted for just about all of them if used at the stated amount.
Mercuric oxide was often recommended as an oxidant in the past. It is now deprecated. It is very toxic, and should be avoided whenever possible. If it must be used, then full safety precautions should be taken (refer to an MSDS), and the used solution must be disposed of in compliance with government regulations to avoid contamination of the environment.
Boiling a solution of hematoxylin with an oxidant invariably causes rapid oxidation and such solutions may be used as soon as cooled. Boiling is not always necessary as it will take place at room temperature over a few days with most oxidizing agents, including sodium iodate. When a solution is needed rapidly, it does enable it to be made available with no negative effects on its staining.
Half oxidation is a term used to describe the partial oxidation of hematoxylin to hematein. The rationale is that during natural ripening the solution becomes usable when a significant portion (i.e. half) of the hematoxylin has been oxidized. These solutions have an extended life because they are usable before full conversion takes place. Throughout their life, oxidation of hematoxylin and further oxidation of already converted hematein continues. They do not become unusable until oxidation proceeds so far that insufficient hematein remains to give acceptable staining.
Chemical half oxidation seeks to emulate this process by adding sufficient oxidant to convert only half of the hematoxylin to hematein. Oxidation will then continue as for a naturally ripened solution. In practice, the limiting factor for use of a solution is more likely to be carryover of alkaline tap water into the solution, neutralizing the acid, and allowing the lake to precipitate. This is why alum hematoxylin solutions can often be rejuvenated by the addition of a small amount of acetic acid. In routine anatomic pathology staining, half oxidation is hardly needed.
| Chemical | Oxidant per gram of dye | |||
|---|---|---|---|---|
| Formula | Maximum | Recommended | Source | |
| Sodium iodate | NaIO3 | 200 mg | 40-150 mg | Lillie, Lynch |
| Mercuric oxide | HgO | 500 mg | 100 mg | Lillie |
| Potassium permanganate | HgO | 177 mg | 175 mg | Lillie |
| Potassium periodate | HgO | 50 mg | 50 mg | Lillie, Lynch |
| Hydrogen peroxide USP | H2O2 | 2.0 mL | – | Lynch |
In addition, calcium hypochlorite (bleach) was used by Anderson, who includes 4 grams to oxidize 2.5 grams hematoxylin in one formula (1.6 grams per gram of hematoxylin), 40 grams to oxidize 5 grams hematoxylin in another (8 grams per gram of hematoxylin), and 5 grams to oxidize 0.5 gram hematoxylin in a third, iron hematoxylin formula (10 grams per gram of hematoxylin). Debiden suggested sodium hypochlorite, using 2 mL of a 5.25% solution of commercial Javex bleach (105 mg chemical) to oxidize 5 grams dye.
Potassium ferricyanide is also included in de Groot’s solution but, although it is a mild oxidant, it is not clear whether this is its function since the solution also contains hydrogen peroxide.
Iron hematoxylin solutions are rarely oxidized directly. Usually, the ferric salts present, being oxidizing agents themselves, do double duty as both mordant and ripener. Nevertheless, some formulae do call for a ripened alcoholic solution to be used. This is particularly so when the mordant and dye are applied separately. It is not an uncommon practice when iron hematoxylin solutions are made by combining two stock solutions just before use, to wait a short time before using the resulting solution and allow the hematoxylin to be oxidized to some extent by the ferric salt mordant first.
Hematoxylin Versus Hematein
The question arises if hematein is the dye and if hematoxylin needs to be oxidized to hematein before it can stain, why not use hematein in the first place?
The answer lies in the difficulty of obtaining satisfactory hematein. It is quite easy to obtain good hematoxylin, and most companies’ products are perfectly OK. Only very rarely is a poor-quality hematoxylin provided.
Hematein is another story, however. It is quite common to purchase unsatisfactory dye, even from reputable providers. Many hematein samples contain only small amounts of the dye, the rest being something else. This is most likely the oxidation products of hematein. These are not dyes and do not stain satisfactorily. They do sometimes give objectionable brown or other off-color tinges to the staining.
The problem is that hematein continues to oxidize to unusable materials. It seems to do this as a powder, and it certainly does it in solution. If we start with hematein, oxidation will start to diminish staining capacity right from the start, shortening the working life of the solution. However, if you get a good sample of hematein, alum-hematein solutions made from it do work well and can be used immediately.
The formulations below specify hematein as the dye.
Keep in mind that almost all solutions could be made with hematein, if the oxidant were omitted. Similarly, solutions specifying hematein can be made with hematoxylin if an appropriate amount of oxidant is added.
Additional Reading
References
- Lillie, R.D., (1954)
Histopathologic technique and practical histochemistry Ed.2
Blakiston, New York, USA. - Gray, Peter. (1954)
The Microtomist’s Formulary and Guide.
Originally published by:– The Blakiston Co.
Republished by:– Robert E. Krieger Publishing Co. - Lynch M.J. et. al.
Medical Laboratory Technology
W. B. Saunders Company, Toronto, Canada - Horobin R W & Kiernan J A, (2002)
Conn’s Biological Stains, 10th ed.
BIOS Scientific Publishers, Oxford, UK






