Formaldehyde
Methanal
CH2O
Before You Begin
Please consult the following guide to safe working with this chemical fixing agent, including how to safely clean up spills.
Safety Note
Formalin can be used quite safely if some simple precautions are followed. Unfortunately, every once in a while an accident occurs, and it is quickly appreciated how important these precautions can be.
The major danger from formalin is asphyxiation. Formaldehyde gas is given off by open formalin and stimulates a choking reflex and an inability to breathe. Spills of surprisingly small amounts of 100% formalin can produce enough formaldehyde in the atmosphere to cause this choking reflex, invariably followed by a deep breath during which more formaldehyde is breathed in causing even greater choking. This choking-breathing cycle is repeated over and over again and can eventually lead to dizziness and collapse in the worst cases.
There are also other effects, caused by the formaldehyde in the atmosphere dissolving into the moisture around the eyes, and into the saliva and nasal secretions. However, these effects are an irritation, but not serious dangers unless continued for long periods.
General handling
Since it is a fixative, it should be self-evident that skin contact should be avoided. Gloves should always be worn, and bare hands or fingers should never be plunged into containers of formalin to retrieve tissue pieces. Doing so can lead to dermatitis. In fact, some people have a sensitivity to formalin and quickly develop a rash on exposure. Those affected in this way should take extra precautions against exposure, as once a sensitivity develops it never goes away. It is preferable for such people to change to another laboratory discipline if possible.
Wear a properly fitted mask with filters specifically designed to remove formaldehyde if working in an atmosphere with detectable amounts of it, such as when removing fixative from tissue prior to disposal or when making up quantities of fixative in bulk. General purpose carbon filters are not appropriate for this, and those designed specifically for formaldehyde are required. In some circumstances, such as during gross sectioning and dictating when exposure can be expected to be limited, an alternative to a mask is a laminar flow hood. It is important to ensure that the airflow is strong enough to remove all formaldehyde fumes. If formaldehyde can be detected then the airflow is not adequate. We appear to be quite sensitive to formaldehyde, and our noses and throats are likely the best detection system in practice.
Neutralizing formic acid
On standing, formalin solutions deteriorate to produce formic acid. Since this can produce acid formaldehyde hematin (formalin pigment) in sections, it is a common practice to neutralize the formic acid. The modern way is to buffer the diluted solution with phosphates (Sorenson’s buffer), but in the past, it was often done by storing the formalin over marble chips. Any formic acid present reacts with the carbonate, producing carbon dioxide and calcium formate, and the formalin remains unacidified. Although this is a simple, cheap and effective procedure it has the potential for a serious problem: the possibility of an explosion when marble chips are put into containers of 100% formalin. As carbon dioxide is generated, the pressure can increase enough to cause the container to explode, showering concentrated formalin (and possibly glass) all over the area. For personal safety, never neutralize concentrated formalin. Add marble to diluted formalin only, and always use a lid that can permit any gas generated to escape.
Carcinogenicity
Formaldehyde is a suspected carcinogen, so exposure should be minimized at all times. The cumulative effects of long-term repeat exposure to high levels of either the fumes or fluids are not well documented. There are anecdotal reports of some pathologists having eye, nose and skin problems following long-term exposure to formalin during gross sectioning. The fumes have either dissolved in their tears and the secretions of mucous membranes, fixing the tissues involved in situ, or repeated skin contact has caused chronic skin damage. It is far better to err on the side of caution and work with formalin in areas that have an adequate air exchange systems. By “adequate”, we mean an air extraction system with enough capacity to remove all traces of the gas so that it is undetectable. Extracting the fumes, of course, does not stop physical contact. Gloves should be worn and sleeves from coats should not drag in fixative (wear short sleeves or roll them up a little).
10% formalin spills
Small volume spills of 10% formalin are usually not too problematic, and usually do not cause distress in breathing. Cleanup while wearing a properly fitted mask with the correct filters attached is usually all that is required. Note the number of times “usually” was used. If there is any difficulty at all, be very cautious. There is nothing to be gained by continuing to work in an objectionable atmosphere.
Note that spills involving large volumes of 10% formalin should be treated as being a significant hazard, since they can be as dangerous as spills of 100% formalin. This is particularly so if in a confined space. If the fumes stimulate choking and a mask is not available, or if the mask does not completely remove the formaldehyde fumes, then this should be treated the same way as a 100% formalin spill, given immediately below.
100% formalin spills
A small spill can be defined as one that does not cause distress in breathing, or one in which the fumes are contained by a properly fitted mask with the correct filters attached. Usually, these are also small in volume as well, being just a few mL. They also pose no significant problem, and may be cleaned up as a 10% formalin spill.
Larger spills pose a significant problem. As little as one liter of 100% formalin can saturate the atmosphere with formaldehyde in a room and make it impossible to breathe properly. In these conditions, the usually available type of mask with canisters designed to remove formaldehyde are useless. A self-contained breathing apparatus (the ones with air cylinders) is required. Most laboratories do not have these, so someone has to hold their breath while working (absolutely to be avoided), or a local hazardous materials team (hazmat) has to be asked to deal with it. In most cases, hazardous materials are dealt with by local fire departments, since using the necessary breathing apparatus is a normal requirement of the job. Call in the Fire Brigade!
It is probably a good idea to limit the decision to call in the fire department to the Supervisor of Anatomic Pathology or the Chief Technologist of the laboratory and others of that level of responsibility, thus diminishing the possibility of unnecessary calls. On the other hand, safety comes first. Better to be embarrassed than dead.
Cleanup
The actual physical cleanup of spilled formalin depends on the type of spill. For small spills, wiping with a paper towel or flushing with water is adequate, depending on how much was spilled. Neutralizer impregnated absorbant towels and pads are available commercially for this purpose. If anything more than a small volume is involved it may cause some problems as the formalin can flow into every nook and cranny when spilled, and missing any just lets the noxious fumes percolate away for some time. More water, rather than less, is always recommended.
Using water is not recommended for other than minimal spills. For larger spills, there are two very effective cleanup systems. One uses urea, and the other ammonia. Either is effective, although the use of ammonia or salts of ammonia is usually faster and less problematic once the formalin smell dissipates.
Urea forms a compound with formaldehyde (urea-formaldehyde resin) which is a sticky solid when fresh, but hardens into a plastic-like hard material after some time. This has to be scraped up and may take some effort to do so. The material is used industrially as an adhesive, which may give some indication of the firmness with which it attaches to benches and floors. Formalin neutralizer granules are available commercially as spill kits, and this is a very convenient way to obtain them.
Ammonia reacts with formaldehyde to produce a non-offensive compound, according to the following:
The ammonial system may be applied in a few different ways, depending on the nature of the cleanup required.
- A 1% aqueous solution of ammonia in a hand spray bottle is convenient for use during gross sectioning. Simply spray the ammonia over the cutting board as needed to control the formalin spill. Small amounts of formalin can be wiped up with paper towels, then the area sprayed with the ammoniated water and wiped again.
- A saturated aqueous solution of an ammonium salt (ammonium sulphate, for instance) is made ahead of time. This solution is poured onto a spill and allowed to react. When the smell dissipates, the mixed fluids are mopped up and the area washed.
- A granulated or powdered ammonium salt (ammonium sulphate, for instance) is mixed into the spill to make a light slurry. This is allowed to react. When the smell dissipates the slurry is collected and the area washed.
Description
In most histology laboratories, formaldehyde in one of its various forms is used as the common fixative. It is inexpensive, easily available, fairly convenient to store, relatively safe when used as directed, forgiving in time, permits use of other fixatives later, allows for long-term storage if changed regularly and isn’t too bad at preserving tissue morphology, although by no means the best at that.
It is usually applied as an aqueous solution made by diluting one volume strong formalin with nine volumes water. Strong formalin contains about 40% formaldehyde gas, so this 1 in 10 dilution contains about 4% of the gas. The diluted solution is referred to as 10% formalin or 4% formaldehyde. On its own or in solutions with other materials added, it is the commonest histological fixative.
View Formaldehyde Terminology Guide >
Strong Formalin
The formaldehyde in strong formalin does not exist as the monomer but as short polymers. When diluted, these revert to the monomer, so solutions of formalin should be made at least a day ahead to allow for this to happen. On standing, the concentrated solution may develop a white precipitate. This is paraformaldehyde, a polymer of formaldehyde. Heating the solution can reverse the polymerization, but heating strong formalin is a potentially dangerous practice (see the safety article referenced above). It is far safer and more practical to ignore it and either filter the diluted solution or allow it to settle. There will be a very slight drop in concentration as a consequence, but it is insignificant and not enough to affect fixation in the slightest.
How it Fixes
Proteins
Formaldehyde causes proteins to cross-link in a meshwork, stabilizing the protein mass and preserving morphology. Baker points out that although it is a strong reducing agent, it fixes by an oxidative reaction, forming methylene bridges between the side amino groups of lysine and glutamine on different protein chains. In the diagram the methylene bridge is red.
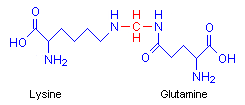
This cross-linking takes some time, and tissues should be treated with simple formaldehyde solutions for a minimum of 48 hours for it to take place. Tissues left for shorter periods than this may show inferior fixation with the likelihood of damage to the preservation by reagents applied after the formalin. In particular, ethanol, used as a dehydrant, may be a problem as it is a fixative in its own right, and may fix proteins that formalin has not fully protected. Unfortunately, ethanol is a poor morphological fixative.
Carohydrates
There are no particular chemical reactions with carbohydrates that would fix them, although the protein part of glycoproteins will be fixed, of course. The cross-linking brought about by formaldehyde does effectively trap glycogen, even though it remains unfixed. It is quite effective at this, although not quite as effective as picric acid. Simple carbohydrates are unaffected.
Lipids
Generally, triglycerides also remain unfixed. Most lipids dissolve during treatment with xylene or other clearant, during paraffin processing, and are not detectable in finished sections. Some phospholipids, however, resist removal by the paraffin process and can be demonstrated. The protein part of lipoprotein may well be fixed, and these too may resist extraction by solvents.
Morphology
Morphological preservation is fair to good. Some shrinkage occurs, and spaces may be seen between elements, but this is not obtrusive. Morphological preservation is at its best when fixation is extended to several days.
Histochemistry
Nucleic acids are not fixed and may be reduced in amount. Most enzymes are inactivated, except for a few if it is used for a short period at 4°C.
Time
Formalin fixes quite slowly, and days are required for fixation to be completed, if it truly ever is. Certainly, 24 hours should be considered minimal. Formalin penetrates tissue fairly quickly but the chemical reaction involved is much slower. This means that penetration by formalin does not indicate the tissue is fixed, i.e. a change in color of the tissue is not a reliable indicator of fixation. This is an important point, since a 1 mm diameter needle biopsy of tissue will be penetrated by formalin within an hour or two and likely change color, but adequate fixation will still require overnight or longer. It is strongly recommended that if rapid fixation for surgical tissue be required, a fixative designed to fix within a short period be used so that processing artifacts can be avoided.
The shortcomings of simple formalin mixtures may be overcome to a limited extent by increasing the temperature, but doing so will reduce the quality of preservation. Elevating the temperature of formalin mixtures moderately can accelerate fixation enough to enable routine overnight processing with generally acceptable results, but doing so may affect special stains and immunohistochemistry. Also, if the temperature is increased too much, heat fixation (cooking) takes place and quality drastically diminishes. In the past, dropping a 1 mm slice of tissue into boiling formalin for 1 minute was often used to fix breast tissue for rapid frozen sections. Some technologists used to leave out the formalin, using plain tap water for the purpose, with much the same results. The point is that too high a temperature is counterproductive.
Simple Solution
Formal saline
By itself, 10% formalin is adequate, but does have some disadvantages. It is not isotonic, and it lyses erythrocytes. To overcome this, it is common to add sodium chloride to it at a concentration of 0.9% w/v. The resulting solution is known as formal saline, and is a standard mixture.
Acid free formalin
On storage, formalin solutions can deteriorate and produce formic acid. This is a problem with bloody tissues, because the formic acid can react with hemoglobin to produce acid formaldehyde hematin, more usually known as formalin pigment. It appears as a brown, doubly refractile, granular deposit dispersed throughout the tissue. Although more common with bloody tissues, in which it can form within a day or so, it is also seen in most tissues stored for a long time in plain formalin mixtures. Removing the acid largely inhibits its formation, and in the past, this was done by storing the formalin over marble chips. However, when the marble is removed the acid is produced again, so tissue stored in the formalin will likely develop formalin pigment over time. This is not usually a problem for tissues that are processed within a day or so. If formalin pigment is present, it may be removed before staining.
Neutral buffered formalin
Perhaps the most popular simple formalin solution is Neutral Buffered Formalin, otherwise known as NBF. It is 10% formalin buffered with sodium dihydrogen phosphate and disodium hydrogen phosphate to pH 7.0, i.e. with Sorenson’s buffer. Using this solution ensures that the pH of the fixative remains constant before and during fixation and, for most purposes, eliminates the problem of formalin pigment except for extremely bloody tissues. It is still possible for it to form during very long-term storage, but changing the fixative every six months or so will restrict that.
Methanol-free formalin
Commercial strong formalin contains some methanol to inhibit polymerization of formaldehyde to paraformaldehyde. This does not usually interfere with fixation, and in the vast majority of applications, 10% formalin made by dilution of commercial strong formalin is completely satisfactory. On those very rare occasions when methanol-free formalin is necessary, it may be made directly from the polymer, paraformaldehyde, which is available commercially as a white powder. To make 100 mL methanol-free 10% formalin, combine 4 grams paraformaldehyde, 100 mL distilled water and a small amount of a base, then raise the temperature above 60°C until the solution clears. This should be done under a fume hood. When it has cooled, other materials, such as buffer salts, may be added.
Aftertreatment
Formalin requires no particular aftertreatment, and fixed tissues may be placed directly into the dehydrant. If using NBF, it is recommended that an alcohol concentration of about 60% or so be used first. The phosphates used to buffer the formalin may precipitate at higher ethanol concentrations and the lower concentration provides an opportunity for the salts to be washed out. Washing formalin fixed tissues can eliminate this problem and, provided the tissue is properly fixed, does no harm. In a surgical service laboratory, however, fixation may not be complete enough for the tissue to withstand extensive washing and nothing more than a quick rinse should be given.
Properly fixed tissues (48 hours or longer) can withstand extensive washing, and doing so can remove much of the formalin attached to the proteins. The tissue never becomes unfixed, of course, but this may be of use for “unmasking” proteins for histochemical methods. It has no practical application for dye staining methods, and is not necessary for most procedures.
Color Restoration
One of the observed effects of formalin fixation is that the color changes, and the life-like appearance of fresh tissue is lost. Formalin fixation permits this color to be restored, but only once. This makes formal saline or NBF the fixatives of choice for tissues to be photographed or for those to be preserved and displayed as medical specimens in glass or methacrylate containers.
For photography, the procedure is to first wash and clean the specimen. It is then soaked in an excess of 60% ethanol until the color has been restored satisfactorily. This takes a little time and the tissue should be checked every five minutes or so until the color is satisfactory. The specimen is then patted dry to remove liquid, which may cause reflections, and photographed. When replaced into fixative or if left in the 60% ethanol for too long, the color changes again and can not be restored a second time.
For mounted specimens, the color is restored in the final mounting fluid (Kaiserling III) by the addition of a small amount of sodium hydrosulphite. If the container is properly sealed, the color restoration is then permanent.
View Color Restoration Guide >
References
- Baker, John R., (1958)
Principles of biological microtechnique
Methuen, London, UK. - Kiernan. J.A., (1999)
Histological and histochemical methods: Theory and practice, Ed. 3
Butterworth Heinemann, Oxford, UK. - Susan Budavari, Editor, (1996)
The Merck Index, Ed. 12
Merck & Co., Inc., Whitehouse Station, NJ, USA






