Amphoteric
Dyes are generally defined along the lines of being colored, aromatic compounds that can ionize. They are thus able to interact with oppositely charged tissue constituents. The groups that are responsible for the ionizing capability are the auxochromes. Amphoteric dyes have both positively chargeable groups and negatively chargeable groups present on the molecule. Depending on the charge actually present, these dyes may interact as either positively charged ions (basic dyes) or negatively charged ions (acid dyes).
The determining factor in compounds of this nature, including amino acids and proteins, is the pH involved. Each such compound has a pH at which the overall charge on the molecule is zero. At this pH, the negatively charged groups and the positively charged groups exactly cancel each other out. This is the isoelectric point, and the compound is considered to form an internal salt at this pH, otherwise known as a zwitterion. At pH levels below the isoelectric point, the positively charged groups are favored, and the compound is considered to be a cation. At pH levels above the isolectric point, the negatively charged groups are favored, and the compound is considered to be an anion.
For most staining methods, the reactions of tissue constituents require pH levels within the range of about pH 2 to pH 10. As it happens, for most dyes containing both positively and negatively charged groups, a pH outside this range is needed to change their overall charge. For that reason, the amphoteric nature of such dyes is theoretical, and is of little practical application, although Edward Gurr proposed a classification system for dyes based on dye auxochromes including classes of amphoteric dyes.
Examples are celestin blue B, a mordant dye with overall negative charge. Also acid fuchsin, which is never considered anything other than an acid dye even though it has amino groups present.
Neutral
Dyes are generally defined along the lines of being colored, aromatic compounds that can ionize. They are thus able to interact with compounds that are oppositely charged. This includes other dyes. In other words, acid dyes can form compounds with basic dyes. Compounds so formed are called neutral dyes. This is not to imply that solutions of these compounds have a pH of 7, merely that both anion and cation are colored.
Many acid and basic dyes can form neutral dyes, but the commonest are probably those that make up the Romanowsky stains. These are derived from the homologues of methylene blue, the azures, and eosins. Particularly azure A or azure B as the cation, and eosin Y or eosin B as the anion. However, most of the dyes in both groups will form neutral dyes, but with inferior staining characteristics.
Other neutral dyes have been recommended from time to time, such as Bowie’s stain for juxtaglomerular granules and Twort’s Gram counterstain using neutral red and light green. The Romanowsky type are the only ones to have gained much popularity.
Making a neutral dye
Generally, neutral dyes are insoluble in water. However, one of the difficulties with making them is that they are often soluble in an excess of either of the initial dyes used to make them. Before making a large amount of a particular neutral dye, it is advisable to determine the amount of the acid dye needed to combine with the basic dye. Remember that many dyes are impure, and may contain substantial amounts of extraneous materials. Some of these materials may also be dyestuffs and could form neutral dyes of their own, but much is inert and merely serves to expand the volume.
To determine how much of each dye to use, begin with the procedure below. Keep in mind that it is a starting point, and the ratios may need to be changed, as may the dye concentrations.
- Make a weak solution of one of the dyes (dye A – say 0.01%)
- Make two solutions of the other dye (dye B):
- One solution should be the same concentration as dye A (0.01%);
- The other solution should be at ten times concentration (0.1%)
- Place 5 mL of dye A into each of ten 15 mL test tubes
- Add 1 mL of dye B1 to the 1st tube, 2 mL to the 2nd etc., for 5 tubes
- Add 1 mL of dye B2 to the 6th tube, 2 mL to the 7th etc. for 5 tubes
- Adjust the volumes to be equal in each tube with distilled water
- Ratios will be (A:B) 1:0.2, 1:0.4, 1:0.6, 1:0.8, 1:1, 1: 2, 1:4, 1:6, 1:8, 1:10
- Mix well, leave overnight
- Next day check for precipitate
- Use the ratios of the dyes in the tube with the heaviest precipitate to calculate how much of each dye to use.
Compounding the neutral dye
- Determine the amount of each dye to use from the above procedure
- Make aqueous solutions of suitable concentration
- Mix the solutions while stirring
- Leave overnight, then filter through Whatman #1 paper
- Wash the filtrate with distilled water to remove unreacted dyes
- Dry at room temperature, protected from dust
- Grind to a powder with a pestle and mortar
- Store in a suitable container
Using the neutral dye
Neutral dyes are not usually soluble in water, but they are soluble in alcohols. For the Romanowsky type of neutral dye, methanol is the alcohol that has been commonly used. This can also be used for non Romanowsky neutral dyes. Ethanol is also quite suitable. For each new dye the optimum concentration has to be determined empirically. A good starting point is to dissolve 0.1 g of the dye into 100 mL of methanol. Trituration may be necessary.
Once the dye has been dissolved into the methanol, do a Romanowsky type stain of a blood smear to get an indication of the staining characteristics. Based on the results, and on subsequent trials, adjust the dye concentration, the times, the degree of dilution and the pH of the diluent as necessary to obtain the optimal stain. Repeat this using tissue sections. Appendix is a suitable tissue. Remember that dehydration may, or may not, remove the stain, so try blotting to dehydrate and clear. Also, try out one of the more complicated, dehydratable Romanowsky techniques. They may be suitable.
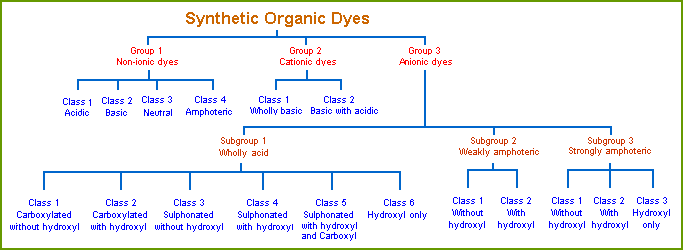
Gurr's Classification System
Edward Gurr proposed a classification system based on auxochromes. He considered auxochromes to be of two types, and coined the term colligator to name them. The two types are colligators and non-colligators. Colligators functioned as ionizing groups, as we now use the term auxochrome. Non-colligators functioned by altering the color of the dye, but did not have ionizing capability. He defined the new term this way:
In Gurr’s classification system, the acid colligators are:
- SO3–
- SO3H
- SO3Na
- OH
- ONa
- OK
- COOH
- COONa
The basic colligators are:
- N+
- NH2
- NH
His system is shown graphically below. Note that Group 1, non-ionic dyes, are the ones with uncharged colligators. Type 2 (cationic) and type 3 (anionic) dyes are charged.
References
- Edward Gurr, (1971)
Synthetic dyes in biology, medicine and chemistry
Academic Press, London, England.






