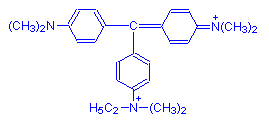
Methyl green
Description
Ethyl green is made from crystal violet by the addition of an ethyl group. Like methyl green, which has a methyl group as the seventh group, crystal violet is common as a contaminant. If it is important to ensure there is no crystal violet present, the solution of ethyl green may be washed with chloroform to extract the crystal violet. Ethyl green is often used, in many cases unknowingly, whenever methyl green is specified and appears to be indistinguishable from it.
The difference in the formula weights probably derives from Conn giving the chloride and bromine salt, and Aldrich giving the zinc chloride salt.
The Merck Index and Aldrich give the name methyl green for this dye. Most histotechnologists know the closely related dye, CI 42585 as methyl green and this dye as ethyl green. The two dyes are very similar, and are probably interchangeable. This is certainly the case as far as the Unna-Pappenheim technique for DNA and RNA is concerned. In many cases, if not all, ethyl green is likely the dye actually provided when methyl green is purchased.
Compare methyl, iodine, and malachite greens.
References
- R. D. Lillie.
Conn’s Biological Stains
Williams & Wilkins, Baltimore, MD., U.S.A. - Aldrich chemical catalogue, 1992
Aldrich Chemical Company, Milwaukee, WI, USA. - Susan Budavari, Editor,
The Merck Index, Ed. 12
Merck & Co., Inc., Whitehouse Station, NJ, USA






