The components involved in histological staining are dyes and proteins. The fundamental process involved is the chemical bonding between the carboxyl groups of one and the amino groups of the other. The most common bonds involved are ionic bonds, although there are exceptions especially in the case of nuclear staining of DNA.
The use of color to identify individual components of tissue sections is accomplished mainly with chemical dyes, although other means are occasionally used. Dyes, however, are the largest group that we can manipulate.
What are Dyes?
In short, dyes are colored, ionizing, aromatic organic compounds.
It must be appreciated that they are individual chemicals, and like all chemicals, they are similar in their reactions to some other chemicals, and distinctly different from others. It may seem that this is a statement of the obvious, but we sometimes appear to view dyes as something other than ordinary chemicals, and I want to stress that the same rules that apply to sodium chloride, acetic acid, benzidine and a host of others also apply to dyes. This includes the possibility that they are toxic. They may be carcinogenic or mutagenic, or harmful to your health in some other way.
Just because we call something by an appealing name and because it has an appealing color, does not make it any less harmful. Handle dyes with care! Put your own safety first!
What is Color?
Dyes are aromatic organic compounds, and as such are based fundamentally on the structure of benzene. To us, benzene appears to be a colorless fluid. In fact, it absorbs electromagnetic radiation just as dyes do, but it does so at about 200 nm so that we do not see it.
The perception of color is an ability of some animals, including humans, to detect some wavelengths of electromagnetic radiation (light) differently from other wavelengths. Normal daylight, or white light, is a mixture of all the wavelengths to which we can respond and some to which we cannot, in particular the infra-red and ultra-violet rays. We respond to wavelengths between about 400-700 nm. When an object absorbs some of the radiation from within that range we see the waves that are left over, and the object appears colored. In reality, this range we see makes up only a very small fraction of the electromagnetic spectrum.
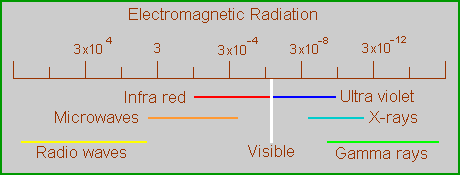
In scientific terms, there is nothing special about the wavelengths in the visible range, other than being the major components of sunlight which are not removed by the earth’s atmosphere. Their special importance is based exclusively on the ability of human retinas to respond to them, and to discriminate between them to a significant degree. These discriminations are what we call color.
Wavelengths just outside the visible range are considered colorless, even though there is no substantive difference between them and the limiting wavelengths inside the range. Some animals (bees, for example) can see these other wavelengths but, because humans do not, we consider them colorless. The point is that color is a subjective phenomenon, and thinking of color as something objective is misleading. For that reason, we should refer to the wavelengths involved rather than describe the human response to them.
When some of the wavelengths found in white light are absorbed, then we see what is left over as colored light. The color that we see is referred to as the complementary color of the color that was removed. For instance, if the red rays are removed from white light, the color we detect is blue-green. Blue-green is complementary to red, and red is complementary to blue-green.
Complementary Colors
| Removed | Observed |
|---|---|
| Violet | Yellow-green |
| Yellow-green | Violet |
| Blue | Yellow |
| Yellow | Blue |
| Cyan | Orange |
| Orange | Cyan |
| Blue-green | Red |
| Red | Blue-green |
| Green | Purple |
| Purple | Green |
The perception of color is merely a human evolutionary adaptation to the absence of some wavelengths in white light. Suppose, however, that the same thing happens outside the range to which our eyes respond. Suppose a chemical removes radiation that has a wavelength about 200 nm, as benzene does. Is this colored?
Well, obviously it is not. There has been no impact on the wavelengths of white light, so it is not colored. However, what has happened to the ultra-violet radiation that was absorbed by the benzene is no different to what happens to the blue radiation that is removed when we look through a solution of acid fuchsin. The difference is solely whether we can detect it visually. Color is not an objective phenomenon. It is the human detection and perception of electromagnetic radiation.
Why are Dyes Colored?
Color in dyes is invariably explained as a consequence of the presence of a chromophore. Since, by definition, dyes are aromatic compounds, their structure includes aryl rings which have delocalized electron systems. These are responsible for the absorption of electromagnetic radiation of varying wavelengths, depending on the energy of the electron clouds. Learn more about why dyes are colored in the guide below.
The Color
The color of the dye is caused by the absorbance of electromagnetic radiation. We have constantly referred to the wavelength that is absorbed in the singular, but a simple scan of a dye solution with a spectrophotometer shows that dyes do not remove a single wavelength. Rather they absorb radiation on either side of the wavelength most completely removed (the absorption maximum). Plotting the wavelength absorbed against the degree of absorbance usually results in a display resembling a bell curve. If any part of this curve is in the visible range, the dye will appear colored.
White light is a mixture of wavelengths. Some of these have a relationship to the energy in the decollated electron cloud of the dye molecule. By the process of resonance, previously described, the electron cloud will respond to the energy contained in that radiation by absorbing it, and removing it from the spectrum. As a consequence, the white light will cease to be white and will display the colors of the wavelengths left over. The transmitted light will have the complementary color to the wavelengths removed.
The Effect
When light illuminates a dye, some of it is absorbed as energy. Since energy is not destroyed, something must then happen. We could use an analogy of heating water – the water’s temperature rises and molecular vibration increases. However, we do not see anything else, as the water just sits there being water.
The same can happen with dyes. We may not observe anything particular as the effect may be at the atomic level. There are several possibilities, however.
- The energy level in the electrons in an unaffected dye is called the ground state. When electromagnetic radiation, as light energy, is absorbed, the electrons become more energized.
- With most dyes, there is then a gradual decay and the electrons return to the ground state. We do not see anything. Nevertheless, something may happen that we do not see. Possibly there is an increase in temperature, or some chemical changes occur that disrupt the dye’s structure and cause it to lose color – fading.
- Another possibility is that the return to the ground state is not gradual, but sudden. If this is accompanied by emission of any residual energy in the form of light, we observe the dye glowing – fluorescence. Since the emitted light must always contain less energy than the absorbed light, as some was used to energize the electrons, the emitted radiation is always at longer wavelengths than the absorbed radiation. By manipulating the light available, we can cause ultra-violet light to be absorbed and visible light to be emitted.
- A third possibility is that the electrons stabilize in their newly energized state. After a passage of time, they then return to the ground state. If this happens gradually, we may observe nothing, with the same possibilities regarding fading and temperature increase as before.
- If return to the ground state happens suddenly, and the residual energy is emitted as light, we once again see the dye glowing – phosphorescence. As with fluorescence, the light emitted is always a longer wavelength than the light absorbed, but the disparity is greater with phosphorescence due to the greater energy consumed in keeping the electrons in the excited state.
Note that the difference between fluorescence and phosphorescence is in whether the electrons stabilize in the excited state before returning to the ground state. With any stability, no matter how long (or short), it is considered phosphorescence.
Dye Classification
Dyes may be structured according to their structure, color index, or other alternative means.
Conclusion
The explanation of the relationship between structure and color depends on the basic atomic structure of the aryl ring, and the shared or delocalized electrons that this atomic arrangement has. The ability to absorb radiation is inherent in this structure. The effect of other atomic configurations is to modify the energy contained in the delocalized electron cloud so that the compound absorbs electromagnetic radiation at a wavelength in the visible range. Some also ionize, enabling the compound to chemically react with ionizing tissue groups.
Color, fading, fluorescence and phosphorescence are all seen to be different effects of the same fundamental process.
References
- Fessenden R J, and Fessenden J S, (1990)
Organic Chemistry, 4th ed.,
Brooks/Cole Publishing Company, Pacific Grove, California - Burstone, M.S.
Enzyme Histochemistry and its application to the study of neoplasms
Academic Press, New York, NY, USA






